How Cellgenic MSCs Revolutionize Regenerative Medicine
Stem Cells are revolutionizing the field of regenerative medicine, due to their intelligence. Once administered into the patient, they are able to identify and target areas of disease and damage. Adimarket’s Mesenchymal Stem Cell Product excretes growth factors, cytokines, and proteins, which all play a key role in the regeneration of tissue. Their anti-inflammatory and immunomodulatory properties mean that it is difficult for them to be rejected by the body. Additionally, they increase blood flow to the vital organs which need it the most.

Many proprietors of MSC products will claim that it is not necessarily important to have a high ratio of viable cells. They claim that it does not matter how many cells are ‘not viable’, or dead, so long as there is a high enough number of viable cells– however, current research has shown that this is not the case. These dead cells are detectable by the immune system, and it is believed that they can create an inflammatory response within the body at the treatment area, which would lower the effectiveness of the regenerative medicine treatments.
This bending of the science is harmful to our industry, which is why knowledgeable purveyors put one thing above all else– consistency. Our cellular concentrations are the same throughout each batch, and we make sure that there is a high ratio of viable cells. All of our samples are independently verified by a third party laboratory, and have been selected for their phenotypic and genotypic profile, characterized for optimum growth and stability. When the proper care is taken, Mesenchymal Stem Cell products have been identified as having the highest output of growth factors and stem cell factors among the current standards of care– as well as properties of angiogenesis, immunomodulation, and the potential for endogenous repair.
Cellgenic has been working for over a decade, constantly reinventing itself and reinforcing the products that we offer with the latest advancements in the field of regenerative medicine. We take every painstaking measure possible to ensure that the cellular samples that our customers use to treat their patients are second to none– this includes the consistent concentrations of our sample, which are the same throughout ensuring that every patient gets the same treatment. We offer the product in 10 million or 30 million live total nucleated cells, where other fabricators would have the same number of total cells. We ensure that every single product that we send out has been tested for low amounts of annexin, which is a cellular protein which serves as a marker for cell death.
All our Mesenchymal Stem Cell products come in 1cc vials cryogenically preserved– they are shipped overnight within the United States, conveniently delivered to your door in the morning. For use, the product is passively thawed between the palms of your hands– and ready to use when your patients are. MSC 10 contains 10 million live cells and is recommended for a single joint, or a small area. However, the MSC pure pro has 30 million live cells,and can be used for larger applications, or for up to three joints in the same patient at the same time.
If you are interested in finding out more about Cellgenic MSCs, you can send an eMail to info@stemcellsgroup.com
- Published in Corporate News / Blog
What is Exosome therapy?
Exosomes are extracellular vesicles (that’s particles that release naturally from a cell that cannot replicate) that are responsible for certain genetic information, otherwise known as Exosomes cell to cell communication. They transport molecules that are essential regulators of intracellular information between close and distant cells.
Exosomes play a vital role in the communication and rejuvenation of all the cells in our body. Despite not being a cell, Exosome has proven to be quite important in maintaining a healthy cellular terrain.
Exosomes are typically made by growing stem cells in culture. Taking the media where they grow and then getting rid of the stem cells. Due to the small size of Exosomes, the media is then ultra-centrifuged to concentrate the exosomes.
Exosomes contain different molecular constituents of their cell origin; these include proteins and RNA. Meanwhile, the Exosomal proteins composition varies with the cell and tissue of the origin; most Exsomes contains an evolutionarily conserved common set of protein molecules.
HOW TO ISOLATE EXOSOME FROM CULTURE STEM?
Exosomes can be isolated from culture stem cell using the method Ultracentrifugation. Often combined with sucrose density gradients, they float the relatively low-density Exosomes from other microvesicles. Cells and larger particles are removed by sequentially increasing the centrifugal forces. These procedures can take up to 30hours and requires an ultracentrifuge, extensive training and specialized equipment.
This method provides highly enriched exosomes but requires specialized training and equipment.
Now, this is how Exosomes works:
Exosomes carry genetic information. Protein and messenger RNA, they can relay this information, letting cells know when to and how to react. This Exosomes cell-to-cell communication is possible due to its unique shape and content.
Exosomes from young stem cells rejuvenate the older cells and assist in calming an overreactive immune system or modulating it to respond in a coordinated and more effective fashion.
WHAT IS EXOSOME THERAPY?
Exosome therapy is one of the hottest areas of regenerative medicine treatment. Researchers have given us valuable insight into the practical functionality of exosomes. While stem cells are usually responsible for the rejuvenation of older cells, they may not be able to supply all the information needed. So supporting Exosome function could have a greater positive effect, by providing a new piece of information to enhance the healing process.
Exosome therapy is a minimally invasive procedure commonly used in orthopedic injuries, for anti-aging and some other degenerative diseases. Exosomes treatment contains growth factors, messenger RNA, micro RNA, cytokines and other biologically active molecules in conjunction with stem cell therapy to speed up healing benefit.
Exosome therapy can be injected into the affected area like in orthopedic injuries or given intravenously for anti-aging.
This Exosome injection is administered directly into the affected area, and the dosing for every patient varies, and there is no set protocol.
Exosomes treatment is gaining popularity recently, due to its ability to transfer molecules from one cell to another via membrane vesicle, therefore influencing the immune system, such as dendrite cells and B cells.
What is exosome therapy used for?
HAIR LOSS: Exosomes injection can be used to regrow hair loss due to its growth factor content. When injected, Exosome will trigger healing, cell stimulation and natural regeneration of these hair follicles. Exosome hair loss therapy is cutting-edge in hair restoration. According to research, there is a positive relationship between Exosomes and Hair loss. Exosomes are ideal for people with thinning hair, excessive shedding or hair loss proper. This is because Exosomes are regenerative cells that can heal, repair, stimulate and restore cells and tissues.
LYME DISEASE: Exosomes may help to regulate processes in the body, and may also be beneficial to patients with Lyme disease. Lyme disease is a very complex disease, which compromises the immune system. Many Lyme patients suffer from dysfunction of the mast cells, increasing their inflammatory response and metabolic function. Incorporating Exosomes treatment in their treatment regimen may help break the inflammatory response and provide the body with necessary cellular information to facilitate healing.
ARTHRITIS: Arthritis as we all know, is the joint inflammation resulting from an autoimmune disease. Although various types of treatment are available to alleviate symptoms, no known therapy has been confirmed effective in reversing the disease progression.
In the field of regenerative medicine today, with the discovery of extracellular microvesicles, especially exosomes, many researchers have been able to offer a more exciting alternative on this subject matter. Exosome arthritis therapy is believed to play a more substantial role in bone and cartilage remodeling.
In a nutshell, Exosomes provides enormous potential in the field of regenerative medicine. You can advance your understanding of Exosomes and its relationship with stem cell in this course. This is an online-course https://www.cellulartherapycourse.com/ where physicians and medical personnel can get insight and deep understanding of this novel science (Exosome).
- Published in Corporate News / Blog
What is Exosome therapy?
Exosomes are extracellular vesicles (that’s particles that release naturally from a cell that cannot replicate) that are responsible for certain genetic information, otherwise known as Exosomes cell to cell communication. They transport molecules that are essential regulators of intracellular information between close and distant cells.
Exosomes play a vital role in the communication and rejuvenation of all the cells in our body. Despite not being a cell, Exosome has proven to be quite important in maintaining a healthy cellular terrain.
Exosomes are typically made by growing stem cells in culture. Taking the media where they grow and then getting rid of the stem cells. Due to the small size of Exosomes, the media is then ultra-centrifuged to concentrate the exosomes.
Exosomes contain different molecular constituents of their cell origin; these include proteins and RNA. Meanwhile, the Exosomal proteins composition varies with the cell and tissue of the origin; most Exsomes contains an evolutionarily conserved common set of protein molecules.
HOW TO ISOLATE EXOSOME FROM CULTURE STEM?
Exosomes can be isolated from culture stem cell using the method Ultracentrifugation. Often combined with sucrose density gradients, they float the relatively low-density Exosomes from other microvesicles. Cells and larger particles are removed by sequentially increasing the centrifugal forces. These procedures can take up to 30hours and requires an ultracentrifuge, extensive training and specialized equipment.
This method provides highly enriched exosomes but requires specialized training and equipment.
Now, this is how Exosomes works:
Exosomes carry genetic information. Protein and messenger RNA, they can relay this information, letting cells know when to and how to react. This Exosomes cell-to-cell communication is possible due to its unique shape and content.
Exosomes from young stem cells rejuvenate the older cells and assist in calming an overreactive immune system or modulating it to respond in a coordinated and more effective fashion.
WHAT IS EXOSOME THERAPY?
Exosome therapy is one of the hottest areas of regenerative medicine treatment. Researchers have given us valuable insight into the practical functionality of exosomes. While stem cells are usually responsible for the rejuvenation of older cells, they may not be able to supply all the information needed. So supporting Exosome function could have a greater positive effect, by providing a new piece of information to enhance the healing process.
Exosome therapy is a minimally invasive procedure commonly used in orthopedic injuries, for anti-aging and some other degenerative diseases. Exosomes treatment contains growth factors, messenger RNA, micro RNA, cytokines and other biologically active molecules in conjunction with stem cell therapy to speed up healing benefit.
Exosome therapy can be injected into the affected area like in orthopedic injuries or given intravenously for anti-aging.
This Exosome injection is administered directly into the affected area, and the dosing for every patient varies, and there is no set protocol.
Exosomes treatment is gaining popularity recently, due to its ability to transfer molecules from one cell to another via membrane vesicle, therefore influencing the immune system, such as dendrite cells and B cells.
What is exosome therapy used for?
HAIR LOSS: Exosomes injection can be used to regrow hair loss due to its growth factor content. When injected, Exosome will trigger healing, cell stimulation and natural regeneration of these hair follicles. Exosome hair loss therapy is cutting-edge in hair restoration. According to research, there is a positive relationship between Exosomes and Hair loss. Exosomes are ideal for people with thinning hair, excessive shedding or hair loss proper. This is because Exosomes are regenerative cells that can heal, repair, stimulate and restore cells and tissues.
LYME DISEASE: Exosomes may help to regulate processes in the body, and may also be beneficial to patients with Lyme disease. Lyme disease is a very complex disease, which compromises the immune system. Many Lyme patients suffer from dysfunction of the mast cells, increasing their inflammatory response and metabolic function. Incorporating Exosomes treatment in their treatment regimen may help break the inflammatory response and provide the body with necessary cellular information to facilitate healing.
ARTHRITIS: Arthritis as we all know, is the joint inflammation resulting from an autoimmune disease. Although various types of treatment are available to alleviate symptoms, no known therapy has been confirmed effective in reversing the disease progression.
In the field of regenerative medicine today, with the discovery of extracellular microvesicles, especially exosomes, many researchers have been able to offer a more exciting alternative on this subject matter. Exosome arthritis therapy is believed to play a more substantial role in bone and cartilage remodeling.
In a nutshell, Exosomes provides enormous potential in the field of regenerative medicine. You can advance your understanding of Exosomes and its relationship with stem cell in this course. This is an online-course https://www.cellulartherapycourse.com/ where physicians and medical personnel can get insight and deep understanding of this novel science (Exosome).
- Published in Corporate News / Blog
The FDA Vs Stem Cell Treatments In The US
The FDA Vs Stem Cell Treatments In The US
Stem cell therapy has become a popular type of treatment for a number of conditions, including to assist in the regeneration of injured tissue or diseases cells in the human body. This therapy is classified as regenerative medicine. Several advancements have already been made in this medical field, and many had experienced positive results when they underwent stem cell therapy to assist in the treatment of degenerative conditions.
While stem cell therapy has been proven to provide an effective protocol in the recovery of stroke, neurological issues, and even to assist in repairing the damage that was dealt by a heart attack, recent reports have caused many to view these treatments in a negative perspective. This is due to the warnings that were recently issued by the FDA. Even though some have experienced side-effects due to the use of stem cell therapy, it is important to note that there are facilities within the US that can provide professional services that help to minimize the risk of these adverse events.
The FDA On Stem Cell Therapy In The United States
The Food and Drug Administration of the United States is involved in the analyzing and approval of medications and medical procedures that may be offered to patients in the country. To date, the FDA has shown some interest in stem cell therapy, providing approval for several clinical trials that looked at how effective this treatment is in providing a regenerating effect on the impact that certain conditions has in the human body. Only one particular treatment that involves stem cell therapy has been officially approved by the Food and Drug Administration, however.
Within the last few months, we have seen several concerning publications made by the FDA regarding stem cell therapies, reducing the hope that some patients might have gained.
On the 25th of June, 2019, the FDA published a statement that described a permanent injunction that will occur with the many stem cell clinics in the country. The FDA has stated that the action taken from their side is to assist in providing a layer of protection for patients, due to the risks involved with undergoing treatments that utilize products that have not gone through any type of approval process.
The specific company that was targeted in this statement and by the actions that were taken by the FDA is known as US Stem Cell Clinic LLC. The court ruled in favor of the FDA and US government earlier in June, finding the defendants, being US Stem Cell Clinic LLC, guilty of the claims that were made against them.
An earlier publication also confirmed that the Federal court has decided to rule the misbranding of the stem cell products released by the US Stem Cell Clinics company as a violation of laws that have been implemented to protect the people of the country.
News publications have also announced that the regulations and guidelines that have now been set out by the FDA might cause a significant decline in the availability of stem cell treatments to patients in the United States. The companies that have been targeted through these particular actions that the FDA has taken are primarily those that have been marketing products that have not gone through approval phases – and these products have been found to put the patient’s health at risk.
The Role Of Stem Cell Therapy In Disease
While some companies have been found guilty of using unapproved and even misleading products on patients, promoted as stem cell therapy, it is important to consider the reality of the situation as well. Stem cell therapy often referred to simply as Cell therapy, has been proven as a successful regime in the treatment of several conditions. Many of the conditions that have shown improvement with the use of this therapy were previously considered difficult, or sometimes even impossible, to effectively treat.
By 2012, the Worldwide Network for Blood and Marrow Transplantation, also known as the WBMT, announced that a total of one million stem cell therapy procedures had been done throughout the world. This was a significant milestone, and these procedures have helped to save a countless number of lives. Just in this report, the WBMT refers back to a case of Marta, a girl from Madrid, who had received stem cell therapy at a young age. This treatment was provided to Marta after she was diagnosed with Leukemia. In 2002, Marta was able to overcome Leukemia, thanks to the cell therapy that was provided to her.
While lymphoma and leukemia are the conditions where patients most frequently seek a clinic that can perform stem cell therapy on them, there are many other conditions that are treated with this procedure today. The perfection of the therapy has led to a treatment that can assist in reducing the effects of over 70 diseases that may otherwise have a significant impact on the human body.
GSCG Expands To Cancun, Meets The Demands Of US Patients
The Global Stem Cells Group had recently announced the opening of a new office that they have decided to establish in Cancun, Mexico. The decision was driven by the increasing demand for cell therapy procedures by patients in the United States and by the fact that there is currently a lack of facilities that are able to provide these individuals with the professional and quality services needed. Doctors specializing in regenerative medicine are also having a hard time fulfilling the needs of patients who wish to consider cell therapy as a potential treatment option.
This is not the only local office that GSCG now operates in Cancun, as the company now has two facilities within this particular location. These offices include a stem cells laboratory, as well as a general medical facility.
Over the years, GSCG has become an established name in the stem cell field, providing thousands of doctors the opportunity to offer cell therapy as a treatment option for patients with degenerative diseases. The use of this therapy may assist in the regeneration of damaged and diseased tissues in the patient’s body.
Even though a previous office had established a presence for GSCG in Cancun, it should be noted that the company announced that the opening of the new office means they have now officially created a permanent presence for the brand in the area.
The GSCG’s new facility in Cancun is equipped with the latest advancements that have been made in the field and provide quality services that ensure patients have a trusted medical facility where their patients can undergo cell therapy as part of a regenerative medicine treatment plan.
At the moment, doctors who are in the field of regenerative medicine within the United States can turn to this facility to offer their patients an additional treatment option, apart from the standard pharmaceutical protocols that have been established. The stem cell laboratory can assist in the analyzing of patient data and also in the process of finding a matching donor for the person who is in need of stem cell treatment.
GSCG’s facility is able to assist with the culturing of expanded autonomous cells, as well as other types of cells that may be utilized in the process of stem cell therapy. A detailed approach is taken, considering the age of the donor, as this would also provide an overview of the autonomous stem cell age. The patient being treated is also taken into close consideration, as there are different methods of introducing these stem cells to the body for enhanced efficacy.
A more aggressive method is often needed in those patients with conditions such as autism, Alzheimer’s disease, and kidney-related conditions. Other conditions can also be treated, such as pulmonary diseases, Parkinson’s disease. In cases of a stroke, the vascular arteries, along with the carotid artery, is targeted with the therapy for a more effective approach to assisting with the regeneration of damaged structures in the patient’s brain. This ultimately leads to a significant improvement in the delivery of stem cells to the patient’s body, which could enhance the overall efficacy expressed by the treatment.
When these procedures are provided to the patient through the GSCG, an appropriate ICU unit is made available to ensure the patient is provided with adequate care during the operation and recovery of the procedure.
The utilization of recent technological advancements at laboratories facilitated by GSCG can also assist in providing a more economical approach to cell therapy. The utilization of allogenic stem cell procedures can be addressed using techniques that will essentially offer a reduction in the costs involved.
Planar technologies may be utilized when the demand is less than one billion cells per annum, which can significantly reduce the costs of the process and, in turn, make these treatments available to patients at a lower price.
The utilization of autologous stem cell transplantation also offers an opportunity for patients to undergo stem cell therapy when a sibling does not provide an ideal match. Cells can be cultivated from the bone marrow and treated with specific drugs in order to yield targeted results. In the case of malignant cells in the body, bone marrow can be treated with monoclonal antibodies or cytotoxic drugs to target such cells when transplanted back into the patient.
Appropriate storage solutions are also taken into consideration to assist with the cultivation process, and to reduce the risk of enzymatic treatment leads to the differentiation of cells into other types. The culture condition becomes crucial for the expansion of stem cells. A PA6 conditioned medium, along with an enclosure of semi-permeable hollow fiber membranes, is one example of how the laboratory can keep certain stem cells from differentiating, while also assuring successful cultivation and expansion.
The GSCG’s Role In The New FDA Warnings
The fact that the FDA has announced the warnings against stem cell therapy products and had even taken action against a relatively popular company in the United States have now caused a limitation in access to these treatments. With a seizure of the services provided by companies that have been affected by the FDA’s actions and the knowledge of the misleading marketing information that was used in the campaigns initiated by these companies, patients might not be sure where to turn to right now.
The same problem is faced by many doctors in the field of regenerative treatments and medicine. Doctors in these fields need to ensure a facility they utilize to allow patients to undergo a treatment like a cell therapy can offer a safe procedure.
This is where the GSCG comes into the bigger picture. With the establishment of a permanent presence in Mexico, doctors are now given access to a facility that has an established history for providing quality stem cell therapy services to patients with qualifying diseases. The company is not only trusted but have been performing stem cell therapy procedures on patients that had led to successful results.
These treatments may help to assist in repairing neurological problems caused by disorders like Parkinson’s disease, stroke, or an injury to the spinal cord. They may also offer new hope to people with diabetes, heart disease, and even those who had previously suffered damage to their cardiovascular system due to a heart attack.
Conclusion
Even though recent publications from the FDA has caused concerns to be raised regarding stem cell therapy, many patients are still interested in undergoing these treatment procedures. A significant number of studies have provided evidence on the efficacy of treating tissue damage caused by various conditions through stem cell therapy. Patients interested in undergoing stem cell treatment are now advised to turn their interest to Stem Cells Centers in Cancun and related regions, where highly specialized and experienced doctors are able to provide a professional service.
- Published in Corporate News / Blog
EXOSOMES
One major aims of regenerative medicine is to replace lost tissue with new cellular material or to improve the regeneration of damaged, malfunctioning, diseases tissue and organs using stem cell transplantation. In view of this, the discovery of Extracellular vesicles (EVs) in the twentieth century have being considered as significant factors in inflammation and immune responses, antigen presentation, immunomodulation, coagulation, tissue regeneration, organ repair, cell-cell communication, senescence, proliferation and differentiation in the body [1]. Extracellular vesicles are believed to be involved in many biological processes and they can be modified to contain specific proteins, genetic lipids, and genetic materials including messenger RNA (mRNA), microRNA (mRNA), and other small non-coding RNAs, and genomic DNA (gDNA) from their progenitor cell.
Extracellular vesicles are classified into two groups which include; exosomes and ectosomes [2]. Exosomes are characterized with cup-shaped morphology, appearred as flattened spheres with diameters ranging from 30 to 150 nm. Similarly, exosomes have a characteristic lipid bilayer which has an average thickness of ~5 nm. [3]. Thus, the lipid components of exosomes include ceramide (sometimes used to differentiate exosomes from lysosomes), cholesterol, sphingolipids, and phosphoglycerides with long and saturated fatty-acyl chains. The exosome is formed or derived from a multivesicular body (MVB) when the MVB fuses with the plasma membrane and is released into the extracellular environment.
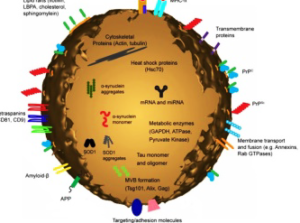
Diagrammatic representation of a medium size Exosome.
Exosomes contain many types of biomolecule, including proteins, carbohydrates, lipids and nucleic acids which vary depending on the EV’s origin, its physiological and pathological state, and even the precise cellular release site. Thus, the protein composition within can also mark the existence of disease pathologies such as inflammatory diseases; however, exosomes also contain a number of common proteins as well as those that participate in vesicle formation and trafficking. Furthernore, exosome plays a role in intercellular communication by carrying proteins and RNAs between neighboring cells or even to distant organs, they also bind to cell membranes through receptor– ligand interaction and mediate antigen presentation, cancer progression etc.
Various techniques have being developed for the isolation of exosomes which includes; ultracentrifugation-based isolation techniques, size-based techniques, precipitation techniques, immune-affinity capture-based techniques, and some novel combination techniques [4]. Exosomes primarily exist in pellets after centrifugation 100000–200000×g and the use of Ultracentrifugation and ultrafiltration can be used to obtain purified exosomes in the laboratory, but this technology is difficult to apply on a large scale [5].
Exosomes can be stored at 4°C for up to 1 week for short-term while it can be stored at -80°C for long-term by suspending it in phosphate buffered saline.
More importantly, growing evidence has also suggested that exosomes play a key role in facilitating tumorigenesis by regulating angiogenesis, immunity, and metastasis. Circulating exosomes in body fluids and blood in particular are potentially non-invasive or minimally invasive biomarkers for early diagnosis and prognosis of various types of cancer.[6] As the first step towards improving human health, exosomes have to be reliably and efficiently isolated from complex biological matrices like blood, urine, and cerebrospinal fluid since they are currently tested as next-generation biomarkers in those body fluids.
In conclusion, it is advantageous to use exosomes for cell based treatments because the use of exosomes can avoid problems associated with the transfer of cells, which may already have damaged or mutated DNA [7]. Also, exosomes are small and can easily circulate through capillaries, whereas the cells used in other cell-based therapies, such as MSCs, are too large to go through capillaries, and thus cannot get beyond first pass capillary beds, such as the lungs.
The level of MSCs in cell-based therapies may quickly diminish after transplant while, exosomes can achieve a higher “dose” than the transplanted MSCs [8]. Similarly, exosomes can also be utilized to tackle toxicity and immunogenicity problems resulting from such biomaterial treatments as nanoparticles [9].
CLINICAL APPLICATION
Ø Immuno-modulatory and anti-inflammatory properties of Exosomes could be the potential biological mechanisms for clinical treatment to promote bone regeneration. [10]
Ø Adipose-derived stem cell-derived exosomes promote fracture healing in animals by binding to polylactic acid-glycolic acid scaffolds.
Ø Exosome can be used for the treatment of chronic kidney disease, type 1 diabetes mellitus, and skin damage [11].
Ø MSCs-derived exosomes have shown therapeutical benefits in stroke and intravenous administration of MSCs-derived exosomes induced an increase of neurogenesis, neurite remodeling, and angiogenesis. [12]
Ø Administration of MSCs-derived exosomes’ has being observed in a traumatic brain injury model by showing an inflammation reduction and good outcomes.[13]
Ø The injection of MSCs-derived exosomes has also been shown to be a possible treatment for spinal cord injury (SCI), by reducing inflammation and by promoting neuro-regeneration in rats after injury [14].
Ø Exosomes can be used as a delivery system of therapeutic signals or drugs due to their low immunogenicity, ability to cross the blood-brain barrier (BBB), and long half-life in circulation.
- Published in Corporate News / Blog
Adimarket Set to Share Innovative Exosomes Products at Upcoming Medical Congresses
The online retailer launched its new line of products in July and will speak with physicians seeking new treatment products and protocols to alleviate patient suffering
MIAMI LAKES, Florida—Adimarket, LLC, an online marketplace for regenerative medicine practitioners and a subsidiary of the Global Stem Cells Group (GSCG), has taken to the road in a series of business trips with the intent of promoting the group’s new line of exosomes, a neonatal-derived stem cells product.
The exosomes products were launched in July 2019 in Buenos Aires, and during the weekend of August 23, Adimarket representatives were in Santiago de Chile to sponsor one of the regenerative medicine industry’s most prolific medical congresses, hosted by the Chilean Society of Aesthetic Surgery. During the event, Mr. Benito Novas, Chief Executive Officer of the Global Stem Cells Group, was on hand to officially launch the exosomes products with a lecture in the congress’ main room.
The Adimarket and GSCG teams will continue their series of business trips to introduce Adimarket’s line of exosomes products to physicians across the globe. Currently, two additional business trips are on the docket where representatives will attend two upcoming medical congresses.
On September 25, 2019 GSCG faculty members will attend the 4th International Congress of Aesthetic Gynecology in Veracruz, Mexico to be held at the Holiday Inn Hotel. One faculty member will speak to the audience about the newest exosomes products and their clinical applications.
The GSCG will also be hosting the International Cell Therapy Symposium on the University of Miami campus in Miami, Florida on October 24, 2019. The event is expected to draw a large attendance of physicians in the regenerative medicine field, and the GSCG has invited more than 10 scientists and practitioners with vast experience in regenerative medicine to host the congress’ educational sessions. At least five of the conference sessions will focus on how exosomes can help people suffering from degenerative diseases
“Adimarket, in conjunction with the Global Stem Cells Group, is pleased to be able to share its newest exosomes products with physicians across the globe practicing regenerative medicine,” said Benito Novas, CEO of the Global Stem Cells Group. “Our goal is to create groundbreaking products and treatment protocols that help physicians deliver innovative treatment options to their patients, helping to alleviate suffering caused by degenerative diseases.”
To learn more about Adimarket and its product offerings, visit https://www.adimarket.net/. To learn more about the Global Stem Cells Group and its efforts in the field of regenerative medicine, visit http://www.stemcellsgroup.com/.
- Published in Press Releases
ISSCA Set to Welcome 13 Speakers at Upcoming Symposium in Miami
The regenerative medicine symposium will be held on the University of Miami campus on October 24, 2019.
MIAMI LAKES, Florida—The International Society for Stem Cell Application (ISSCA) has announced its lineup of speakers for its upcoming Regenerative Medicine Symposium. The symposium will be held on the University of Miami campus in the Donna E. Shalala Student Center on October 24, 2019 and features an impressive lineup of global authorities in regenerative medicine.
ISSCA is a global leader in stem cells research, applications, and education, partnering with major global institutions and locations worldwide to host its independent medical congresses. This year’s symposium hosted on the University of Miami campus will feature industry experts from seven countries, including Spain, Argentina, Bolivia, Colombia, Ecuador, Mexico, and the US. Some notable speakers include the following:
- Dra. Silvina Pastrana from Argentina, who serves as the Medical Director of the Stem Cells Center Buenos Aires. Dra. Pastrana will discuss her experiences in treating patients who suffer from osteoarthritis conditions.
- Dr. Pedro Sanchez hails from Colombia and is a highly regarded regenerative medicine and orthopedic specialist. He currently practices in Bogota where he offers stem cells treatments for people who suffer from orthopedic conditions.
- Dr. Miguel Guillermo Garbe of Spain serves as the president of the country’s Regenerative Medicine Association. He currently leads a seminal investigation on how to increase cardiological function in patients who have suffered from strokes.
- Dr. Roberto Blum from Ecuador has worked in the stem cells field for over 10 years and will share his industry experience with attendees.
- Dr. Victor Pereyra, an Argentinian neurosurgeon, will lecture on how ozone therapy and stem cells could help patients suffering from spinal injuries.
“The symposium will provide an informative day where physicians can learn from global leaders in the stem cells industry with the hopes of bringing new knowledge and treatment options back to their practices for their patients suffering from degenerative diseases.”
To learn more about the ISSCA and its Miami symposium, visit
http://www.stemcellsgroup.com/.- Published in Press Releases
GSCG Meets Demands of Patients Seeking Stem Cells Treatment with New Facilities
The group’s new permanent office in Cancun will help streamline the patient experience by providing holistic support throughout the treatment process.
MIAMI LAKES, Florida—The Global Stem Cells Group (GSCG) today announced that it will be opening a permanent office in Cancun, Mexico. The decision to expand its corporate presence into Cancun was based on the rising demand for patients seeking stem cells treatments at the group’s facilities already established in Cancun. The GSCG maintains two regenerative medicine operations in Cancun, which include medical facilities and a stem cells laboratory.
GSCG’s Cancun facilities provide state-of-the art options for patients seeking stem cells treatments that are currently not available in the US. While these protocols and products are currently unavailable within the US’s borders due to regulations, the Cancun center has still adopted the FDA’s frameworks for safe and effective regenerative medicine products and therapies. Guided by these protocols, physicians at the center deliver safe and effective stem cells therapies and products that can help patients suffering from degenerative diseases recover more quickly than with traditional treatment protocols.
With the establishment of a permanent office presence in Cancun, the GSCG seeks to provide a seamless holistic experience for patients seeking treatment at its facilities. With its office located near its medical center and laboratory, the GSCG team can more closely monitor the treatment process from the time the patient arrives at the Cancun airport to the time their treatment is complete and they return to the US.
“The Global Stem Cells Group is pleased to establish a permanent presence in Cancun with the opening of this new office location,” said Benito Novas, CEO of the Global Stem Cells Group. “By expanding our presence, we will be able to open up additional treatment opportunities for physicians in the US who may be limited by FDA regulations in ensuring their patients receive the critical stem cells therapies they need. US patients looking for relief from regenerative diseases are welcome at our Cancun center and will appreciate our state-of-the art facilities and top-notch care.”
To learn more about the Global Stem Cells Group and its latest efforts, visit http://www.stemcellsgroup.com/.
- Published in Press Releases
Global Stem Cells Group to Break Ground on New Lab in Mexico
The new Stem Cells Lab is set to break ground in 2020, providing affordable regenerative medicine products to Latin America and the US
MIAMI LAKES, Florida—The Global Stem Cells Group today announced that it will be expanding its research and production efforts with a new lab in Mexico. The lab is the group’s first facility of its kind and will produce neonatal tissue-derived products such as exosomes, cord blood derived stem cells, and amniotic fluid derived products. Groundbreaking is slated for January 2020.
The Global Stem Cells Group is a multi-disciplinary community of scientists and physicians collaborating to treat diseases and lessen human suffering through science, technology, and regenerative medicine. To further the group’s mission, the new lab will fill a much-needed research and production niche in the regenerative medicine field and will be especially beneficial to physicians practicing in Latin America and the United States.
The lab will strictly adhere to all Good Manufacturing Practice (GMP) standards. Although the lab will be located in Mexico, all products will be procured and processed according to the standards and regulations set forth by the American Association of Tissue Banks (AATB) and the United States Food and Drug Administration (FDA). All tissue will come from local donors, with the lab strictly adhering to donor ethics and the FDA’s approved serological screening policies.
In addition to producing neonatal tissue derived products, the lab will also facilitate clinical trials to explore the safety and efficacy of new regenerative medicine products before they launch. The lab will also serve as a teaching and learning center for doctors and scientists across the globe looking to learn more about stem cells protocols.
“The Global Stem Cells Group is committed to facilitating stem cell research and medicine across the globe,” said Benito Novas, CEO of the Global Stem Cells Group. “Our new lab in Mexico will allow us to make good on this mission by delivering more affordable neonatal tissues derived products to doctors across Latin America and the US. As a result, more patients will find relief from debilitating degenerative diseases.”
To learn more about the Global Stem Cells Group and its latest efforts, visit http://www.stemcellsgroup.com/.
- Published in Press Releases
Adimarket Releases New Exosomes Product that Delivers Therapeutic Promise
AdimarketTM Flow uses exosomes in compliance with US regulations to provide innovative treatment options to those suffering from degenerative diseases.
MIAMI LAKES, Florida—Adimarket, LLC, a subsidiary of the Global Stem Cells Group is a leading distributor of products for regenerative medicine providers, has announced a new stem cells product, exosomes, to its product portfolio. The product is available online via Adimarket’s website at https://www.adimarket.net/ and comes in a 1 mL vial and is available for 24-hour delivery after orders placed from any US city.
Exosomes contain a variety of genetic materials, such as mRNAs and microRNAs (miRNAs), which imply they may play a critical role in cell-to-cell communication. AdimarketTM Flow is procured and processed according to US standards and regulations established by the American Association of Tissue Banks (AATB) and the United States Food and Drug Association (FDA).
Exosomes are currently non-reactive and FDA approved.
Exosomes provide an exciting new treatment option for those suffering from degenerative diseases. As a next-generation therapy option, exosomes work by isolating the beneficial signals released by stem cells and use them rather than the stem cells themselves. Using exosomes as a viable therapeutic option makes sense, as they cause other cells to react and change their behavior accordingly. Research and application demonstrate that there is incredible therapeutic potential within extracellular vesicles, particularly exosomes.
Physicians exploring exosomes as a therapeutic option should familiarize themselves with their components and signal deliveries. Components of exosomes include MSCs, cytokines, chemokines, growth factors and hyaluronic acid (HA). They are able to deliver the following signals to target cells: anti-apoptotic, anti-fibrotic, pro-angiogenic and pro-differentiation.
Adimarket, LLC is a leading distributor of products for practitioners in the regenerative medicine field. The online market offers a vast portfolio of goods, ranging from office equipment to cellular- and tissue-based products. The Global Stem Cells Group, which owns Adimarket, continually develops and launches new products specifically for regenerative medicine practitioners to assure physicians have all they need to practice in their field safely and effectively.
To learn more about AdimarketTM Flow and all of Adimarket’s regenerative medicine products, visit https://www.adimarket.net/product/cellgenic-flow-1ml-exosomes/.
- Published in Press Releases
- 1
- 2



![Adrian-Learning-7[1]](https://stemcellcenter.net/wp-content/uploads/2019/11/Adrian-Learning-71-460x260_c.png)
![WhatsApp-Image-2019-07-30-at-12.02.45-PM[1]](https://stemcellcenter.net/wp-content/uploads/2019/11/WhatsApp-Image-2019-07-30-at-12.02.45-PM1-460x260_c.jpeg)
![IMG_UMsign0700_Stock02_M_2_1_57A6MDOD_L280954504[1]](https://stemcellcenter.net/wp-content/uploads/2019/11/IMG_UMsign0700_Stock02_M_2_1_57A6MDOD_L2809545041-460x260_c.jpg)
![ameri1[1]](https://stemcellcenter.net/wp-content/uploads/2019/11/ameri11-460x260_c.jpg)
![WhatsApp-Image-2019-08-02-at-9.40.58-AM[1]](https://stemcellcenter.net/wp-content/uploads/2019/11/WhatsApp-Image-2019-08-02-at-9.40.58-AM1-460x260_c.jpeg)
![DJI_20181127_125551[1]](https://stemcellcenter.net/wp-content/uploads/2019/11/DJI_20181127_1255511-460x260_c.jpg)