Global Stem Cells Group Announces Manufacturing Phase of Progenikine™ SVF Closed System
Global Stem Cells Group has begun the manufacturing phase of Progenikine™, a new SVF closed system kit utilizing EmCyte technology, containing all the elements necessary to process adipose tissue and obtain stromal vascular fraction in a sterile environment.
MIAMI, March 31, 2016—Global Stem Cells Group, Inc. has announced that Progenikine™, its new and approved SVF closed system kit using EmCyte technology, is in the manufacturing phase and will be available to physicians in July 2016. The Progenikine kit contains all the elements necessary to process adipose tissue and obtain stromal vascular fraction (SVF) in a closed environment.
Adipose derived stem cells (ASCs) are used by physicians for a variety of indications. Most commonly, ASCs are  isolated at the point of care from lipoaspirate (derived from liposuction) tissue as the stromal vascular fraction (SVF), harvested from the patient and immediately administered to the patient as an injection, or used to enrich fat grafts. Isolation of ASCs from adipose tissue is a relatively simple process performed routinely in cell biology laboratories, but isolation at the point of care for immediate clinical administration requires special methodology to prevent contamination, ensure integrity of the clinical procedure, and comply with regulatory requirements.
isolated at the point of care from lipoaspirate (derived from liposuction) tissue as the stromal vascular fraction (SVF), harvested from the patient and immediately administered to the patient as an injection, or used to enrich fat grafts. Isolation of ASCs from adipose tissue is a relatively simple process performed routinely in cell biology laboratories, but isolation at the point of care for immediate clinical administration requires special methodology to prevent contamination, ensure integrity of the clinical procedure, and comply with regulatory requirements.
Developed in conjunction with Patrick Pennie, Emcyte CEO, and and Maritza Novas Director of Research and Development for Global Stem Cells Group, Progenikine  fuses elements from Emcyte systems with the Global Stem cells Group SVF protocols.The kit can provide a low cost, rapid and simple alternative to traditional methods of isolating ASCs, particularly when smaller quantities are needed.
fuses elements from Emcyte systems with the Global Stem cells Group SVF protocols.The kit can provide a low cost, rapid and simple alternative to traditional methods of isolating ASCs, particularly when smaller quantities are needed.
“The Progenikine kit is the newest product designed to help Global Stem Cells Group’s mission to provide accessible products to our member clients, ensuring that more patients will be able to gain access to stem cell therapies,” says Benito Novas, GSCG CEO.
For more information on Global Stems Cell Group, visit the Global Stem Cells Group website,email bnovas(at)stemcellsgroup(dot)com, or call +1 305 560 5337.
About Global Stem Cells Group:
Global Stem Cells Group, Inc. is the parent company of six wholly owned operating companies dedicated entirely to stem cell research, training, products and solutions. Founded in 2012, the company combines dedicated researchers, physician and patient educators and solution providers with the shared goal of meeting the growing worldwide need for leading edge stem cell treatments and solutions. With a singular focus on this exciting new area of medical research, Global Stem Cells Group and its subsidiaries are uniquely positioned to become global leaders in cellular medicine.
Global Stem Cells Group’s corporate mission is to make the promise of stem cell medicine a reality for patients around the world. With each of GSCG’s six operating companies focused on a separate research-based mission, the result is a global network of state-of-the-art stem cell treatments.
About Emcyte:
Fort Myers, Florida-based EmCyte Corporation is a leader in autologous cellular biologics with the GenesisCS Component Concentrating Systems. These systems provide patients with the best opportunity for rapid recovery and provide practitioners with the most advanced clinical point of care experience. EmCyte systems are developed to meet every clinical requirement, giving the physician better clinical choices. EmCyte devices have been independently reviewed and show to produce buffycoat concentrations of 6x to greater than 10x baseline in 7mLs, with yields ranging from 70 percent to greater than 90 percent
EmCyte technology allows for the safe extraction of concentrated platelets and other regenerative cell types from the patient’s own blood. These cells are then re-suspended in a small volume of the patient’s blood plasma and then applied to the treatment site.
###
To view this press release live online, click here
- Published in Press Releases
Our Friend MSCs (Mesenchymal Stem Cells)—Bringing New Life to Old Bones
Researchers from the University of Toronto and The Ottawa Hospital were looking to see if mesenchymal stem cells (MSCs) might treat osteoporosis. MSCs are multipotent stromal cells that can differentiate into a variety of cell types, including: bone cells (osteoblasts), cartilage cells (chondrocytes), muscle cells (myocytes) and fat cells (adipocytes).
Faulty MSCs are the culprits behind osteoporosis; after injecting healthy MSCs into mice with the affliction that causes bones to become weak and brittle, researchers were hoping for a general increase in the mice’s bone health. Instead, they were surprised (and probably very excited) to discover after six months—a quarter of a mouse’s life span—that healthy, functioning bone had replaced the damaged osteoporotic bone. The bone structure in the little creatures, which had been severely compromised by osteoporosis, had been restored to a normal, healthy state! The healthy mesenchymal stem cells did what they were born to do. They became bone cells and went to work, much like the restoration of an old building at the hands of architects and laborers, only without the scaffolds and noise. MSCs work very quietly.
Researchers are hoping that these findings could lead to a new way of treating osteoporosis in humans, or even delay its onset indefinitely.
Stem cell researchers have known for some time that MSCs can boost the regeneration of bone, and in fact a test group of elderly patients in the U.S. who suffer from osteoporosis have already received MSC injections as part of an ancillary trial. The research team is preparing to to examine their blood samples to see if biological markers indicate an improvement in bone growth and bone reabsorption.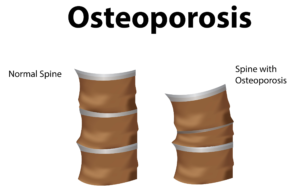
Depending on the outcome of those blood tests, larger trials involving human patients could follow within the next 5 years.
In addition to working quietly and therefore not waking you to the sound of a jackhammer at 7 a.m., there are other cool qualities to MSCs. For instance, they are “a heterogeneous population of musculoskeletal progenitors (another name for adult stem cells) that includes skeletal stem cells (SSCs).” An added perk is that they can be transplanted between individuals without the need to be matched, and without the risk of rejection.
MSCs are awesome.
Globally, more than 200 million people are living with either postmenopausal osteoporosis—known as type 1 osteoporosis, which affects mainly women, or age-related type 2 osteoporosis, which affects both men and women.
With type 2 osteo porosis, there is a reduction in the inner structure of the bone. The bone becomes thinner and less dense, and it can no longer function properly.
porosis, there is a reduction in the inner structure of the bone. The bone becomes thinner and less dense, and it can no longer function properly.
Worldwide, type 2 osteoporosis leads to around 8.9 million bone fractures annually. Hip fractures are among the most common fractures related to osteoporosis, which can lead to disability and even death in elderly patients.
Currently, Teriparatide (brand name Fortéo) is the only drug available to treat type 2 osteoporosis, and its effectiveness lasts for only two years.
The senior author of the study, titled Systemic Mesenchymal Stromal Cell Transplantation Prevents Functional Bone Loss in a Mouse Model of Age-Related Osteoporosis, and published March 17, 2016, is William Stanford, Ph.D., a senior scientist at The Ottawa Hospital Research Institute and a professor at the University of Ottawa. Previous research led Stanford to discover the association between defects in MSC and age-related osteoporosis in mice.
The study’s co-author, John E. Davies, Ph.D., D.Sc., is a professor at the University of Toronto’s Institute of Biomaterials and Biomedical Engineering, The study’s findings are published in the current issue of Stem Cells Translational Medicine.
- Published in Corporate News / Blog
How Stem Cell Therapies Can Help Heal Sports Injuries
Continuing our recent discussion of stem cell therapies for sports injuries, the use of mesanchysmal stem cells (MSCs) in orthopedic medicine can help in the repair of damaged tissue by harnessing the healing power of undifferentiated cells that form all other cells in our bodies. The process involves isolating these stem cells from a sample of your blood, bone marrow or adipose tissue (fat cells), and injecting it into the damaged body part to promote healing. Platelet-rich-plasma (PRP), a concentrated suspension of platelets (blood cells that cause clotting of blood) and growth factors, is also used to assist the process of repair.
Below are some examples of injuries and areas of research involving the use of mesenchymal stem cells (MSCs), which are (adult) tissue stem cells that are not only able to produce copies of themselves, but also able to divide and form bone, cartilage, muscle, and adipose (fat) stem cells when cultured under certain conditions:
Cartilage Damage
Cartilage has long bee n considered as an ideal candidate for cell therapy as it is a relatively simple tissue, composed of one cell type, chondrocytes, and does not have a substantial blood-supply network. Of particular interest to researchers is repair of cartilage tissue in the knee, also called the meniscus of the knee. The meniscus is responsible for distributing the body’s weight at the knee joint when there is movement between the upper and lower leg. Only one third of meniscus cartilage has a blood supply, and as the blood supply allows healing factors and stem cells attached to the blood vessels (called perivascular stem cells) to access the damaged site, the meniscus’s natural lack of blood supply impairs healing of this tissue. Damage to this tissue is common in athletes, and is the target for surgery in 60 percent of patients undergoing knee operations, which usually involves the partial or complete removal of the meniscus, which can lead to long-term cartilage degeneration and osteoarthritis.
n considered as an ideal candidate for cell therapy as it is a relatively simple tissue, composed of one cell type, chondrocytes, and does not have a substantial blood-supply network. Of particular interest to researchers is repair of cartilage tissue in the knee, also called the meniscus of the knee. The meniscus is responsible for distributing the body’s weight at the knee joint when there is movement between the upper and lower leg. Only one third of meniscus cartilage has a blood supply, and as the blood supply allows healing factors and stem cells attached to the blood vessels (called perivascular stem cells) to access the damaged site, the meniscus’s natural lack of blood supply impairs healing of this tissue. Damage to this tissue is common in athletes, and is the target for surgery in 60 percent of patients undergoing knee operations, which usually involves the partial or complete removal of the meniscus, which can lead to long-term cartilage degeneration and osteoarthritis.
Recently, researchers increased their focus on the use of MSCs for treatment of cartilage damage in the knee. Some data from animal models suggest that damaged cartilage undergoes healing more efficiently when MSCs are injected into the injury, and this can be further enhanced if the MSCs are modified to produce growth factors associated with cartilage. It has been shown that once the MSCs are injected into the knee they attach themselves to the site of damage and begin to change into chondrocytes, promoting healing and repair. A small number of completed clinical trials in humans using MSCs to treat cartilage damage have reported some encouraging results, but these studies used very few patients, making it difficult to accurately interpret the results. There are currently a number of ongoing trials using larger groups of patients, and the hope is that these will provide more definite information about the role MSCs play in cartilage repair.
Tendinopathy
Tendinopathy relates to injuries that affect tendons – the long fibrous tissues that connect and transmit force from  muscles to bones. Tendons become strained and damaged through repetitive use, making tendinopathy a common injury among athletes. Tendinopathy has been linked to 30 percent of all running-related injuries, and up to 40 percent of tennis players suffer from some form of elbow tendinopathy or “tennis elbow.” Damage occurs to the collagen fibers that make up the tendon, and this damage is repaired by the body through a process of inflammation and production of new fibers that fuse together with the undamaged tissue. However, this natural healing process can take up to a year to resolve, and results in the formation of a scar on the tendon tissue, reducing the tendon’s natural elasticity, decreasing the amount of energy the tissue can store and resulting in a weakening of tendon.
muscles to bones. Tendons become strained and damaged through repetitive use, making tendinopathy a common injury among athletes. Tendinopathy has been linked to 30 percent of all running-related injuries, and up to 40 percent of tennis players suffer from some form of elbow tendinopathy or “tennis elbow.” Damage occurs to the collagen fibers that make up the tendon, and this damage is repaired by the body through a process of inflammation and production of new fibers that fuse together with the undamaged tissue. However, this natural healing process can take up to a year to resolve, and results in the formation of a scar on the tendon tissue, reducing the tendon’s natural elasticity, decreasing the amount of energy the tissue can store and resulting in a weakening of tendon.
MSCs have the ability to generate cells called tenoblasts that mature into tenocytes. These tenocytes are responsible for producing collagen in tendons. This link between MSCs and collagen is the focus for researchers investigating how stem cells may help treat tendinopathy. Substantial research has been carried out on racehorses as they suffer from high rates of tendinopathy, and the injury is similar to that found in humans. Researchers discovered that by injecting MSCs isolated from an injured horse’s own bone marrow into the damaged tendon, recurrence rates were almost cut in half compared to horses that receive traditional medical management for this type of injury. A later study by the same group showed the MSCs improved repair, resulting in reduced stiffness of the tissue, decreased scarring and better fusion of the new fibers with the existing, undamaged tendon. It is not yet clear if these results are due to MSCs producing new tenocytes or their ability to modulate the environment around the tendinopathy, as described above. These promising results paved the way for the first pilot study in humans.
Bone Repair
 Bones are unique in that they have the ability to regenerate throughout life. Upon injury, such as a fracture, a series of events occur to initiate healing of the damaged bone. Initially there is inflammation at the site of injury, and a large number of signals are sent out. These signals attract MSCs, which begin to divide and increase their numbers. The MSCs then change into either chondrocytes, the cells responsible for making a type of cartilage scaffold, or osteoblasts, the cells that deposit the proteins and minerals that comprise the hard bone on to the cartilage. Finally these new structures are altered to restore shape and function to the repaired bone. A number of studies carried out in animals have demonstrated that direct injection or infusing the blood with MSCs can help heal fractures that previously failed to heal naturally. However, as was the case with tendinopathy, it is not yet clear if these external MSCs work by generating more bone-producing cells or through their ability to reduce inflammation and encourage restoration of the blood supply to injured bone, or both.
Bones are unique in that they have the ability to regenerate throughout life. Upon injury, such as a fracture, a series of events occur to initiate healing of the damaged bone. Initially there is inflammation at the site of injury, and a large number of signals are sent out. These signals attract MSCs, which begin to divide and increase their numbers. The MSCs then change into either chondrocytes, the cells responsible for making a type of cartilage scaffold, or osteoblasts, the cells that deposit the proteins and minerals that comprise the hard bone on to the cartilage. Finally these new structures are altered to restore shape and function to the repaired bone. A number of studies carried out in animals have demonstrated that direct injection or infusing the blood with MSCs can help heal fractures that previously failed to heal naturally. However, as was the case with tendinopathy, it is not yet clear if these external MSCs work by generating more bone-producing cells or through their ability to reduce inflammation and encourage restoration of the blood supply to injured bone, or both.
Brain injury in sports
There is mounting evidence that those taking part in sports where they are exposed to repetitive trauma to the head and brain are at a higher risk of developing neurodegenerative disorders, some of which are targets for stem cell treatments. For example, it has been reported that the rate of these diseases, like Alzheimer Disease, were almost four times higher in professional American football players compared to the general population. While the cause of this disease is not yet clear, it is associated with abnormal accumulation of proteins in neural cells that eventually un dergo cell death and patients develop dementia. Researchers have attempted a number of strategies to investigate treatments of this disease in mice, including introducing neural stem cells that could produce healthy neurons. While some of these experiments have demonstrated positive, if limited, effects, to date there are no stem cell treatments available for Alzheimer’s Disease.
dergo cell death and patients develop dementia. Researchers have attempted a number of strategies to investigate treatments of this disease in mice, including introducing neural stem cells that could produce healthy neurons. While some of these experiments have demonstrated positive, if limited, effects, to date there are no stem cell treatments available for Alzheimer’s Disease.
Boxers suffering from dementia pugilistica, a disease thought to result from damage to nerve cells, can also demonstrate some symptoms of Parkinson’s Disease (among others). In healthy brains, specialized nerve cells called dopaminergic neurons produce dopamine, a chemical that transmits signals to the part of the brain responsible for movement. The characteristic tremor and rigidity associated with Parkinson’s Disease is due to the loss of these dopaminergic neurons and the resulting loss of dopamine production. Researchers are able to use stem cells to generate dopaminergic neurons in the lab that are used to study the development and pathology of this disease. While a recent study reported that dopaminergic neurons derived from human embryonic stem cells improved some symptoms of the disease in mice and rats, stem cell based treatments are still in the development phase.
- Published in Corporate News / Blog
Could stem cells repair the damaged brain in Alzheimer’s?
Stem cell therapies may hold the cure to Alzheimer’s, although so far that cure has been elusive. People who suffer from Alzheimer’s disease experience disorientation regarding time and place, changes in mood, personality and behavior, memory loss, difficulty solving problems or planning, and difficulty writing or performing other routine and familiar tasks. This progressive and irreversible brain disorder may affect judgment, initiative and social life, and can lead to physical symptoms such as vision problems.
Alzheimer’s affects mostly people aged 70 years and above, and is more common in women. It is the main risk factor for dementia among the elderly.
There is no known cure for Alzheimer’s. Conventional treatments, both drug-based and non-drug strategies, may help with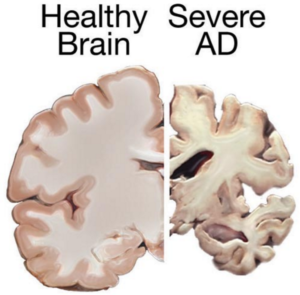 cognitive and behavioral symptoms, but have little to no effect on the disease’s development over the long term. Current medications can’t stop Alzheimer’s from progressing, but they can temporary lessen symptoms like confusion and memory loss.
cognitive and behavioral symptoms, but have little to no effect on the disease’s development over the long term. Current medications can’t stop Alzheimer’s from progressing, but they can temporary lessen symptoms like confusion and memory loss.
Although there have been attempts to find a remedy for Alzheimer’s, and despite the fact that scientists have managed to effectively treat lab animals with drug-based treatments, no animal model has managed to truly mimic its symptoms as they manifest in humans. Remedies that worked in lab animals have failed to work in humans; for this reason, scientists decided to try a different approach by exploring the possibilities of stem cells therapies in Alzheimer’s treatments.
Can Stem cells develop new Alzheimer’s treatments?
Alzheimer’s disease affects neurons in all parts of the brain, and the complexity of this condition makes it difficult to create a model that perfectly mimics its manifestations. At least in theory, stem cells could be used for treating this condition by transplanting neural stem cells into the patient’s brain in an attempt to generate healthy new neurons to replace dead and damaged neurons. It remains unclear whether the brain would be able to integrate the transplanted cells, and if the neural stem cells are able to travel to the damaged areas.
Another great challenge is producing the different types of neurons needed to replace the damaged cells, and to find a way to stimulate the renewal of the lost connections between neural cells. Even if the transplanted cells survive and find their way to the damaged areas, they might become damaged by proteins that build up in the brain—the same proteins that cause the disease in the first place, which means any effects of a stem cell transplant could be only temporary.
 A different approach would be to use stem cells for delivering neurotrophins to the brain. Neurotrophin proteins support the growth and survival of neurons, but in patients with Alzheimer’s, they’re produced in amounts too low to contribute such support. Neural stem cells can produce such cells, and in mice tests this method did prove helpful; scientists observed some improvements in memory in mice treated with stem cells.
A different approach would be to use stem cells for delivering neurotrophins to the brain. Neurotrophin proteins support the growth and survival of neurons, but in patients with Alzheimer’s, they’re produced in amounts too low to contribute such support. Neural stem cells can produce such cells, and in mice tests this method did prove helpful; scientists observed some improvements in memory in mice treated with stem cells.
Mesenchymal stem cells could also be used for treating Alzheimer’s—not to replace damaged neurons, but to heal them. Mesenchymal stem cells may exert anti-inflammatory effects and may help ameliorate the symptoms of Alzheimer’s, but there’s no study at the moment to prove their safety or effectiveness in this condition [3].
Although clinical trials and studies on Alzheimer’s disease treatment to date have a high failure rate, the use of stem cells may be helpful for studying the behavior of brain cells damaged by the condition, as well as for testing various therapeutic approaches and predicting which treatment may help Alzheimer’s patients.
Researchers from the Harvard Stem Cells Institute took skin cells from Alzheimer’s patients and reprogramed them to create induced pluripotent stem cells (iPSC); obtained cells were grown in special lab conditions and were found to release the same proteins that form plaque in Alzheimer’s patients [2]. This may give scientists the opportunity to study the behavior of Alzheimer’s-affected cells and to search for and test new remedies.
Asian scientists managed to turn human fibroblasts into neuronal cells using a chemical cocktail of small molecules [6]. These findings may provide an alternative strategy for modeling the neurodegenerative disorder, which may help scientists understand the mechanisms behind this condition. It may also play an important role in identifying new stem cell based treatments.
References
- http://www.eurostemcell.org/factsheet/alzheimer%E2%80%99s-disease-how-could-stem-cells-help
- http://hsci.harvard.edu/news/alzheimer%E2%80%99s-dish
- http://www.ipscell.com/2012/05/can-stem-cells-be-used-to-treat-alzheimers-disease/
- http://www.sciencedirect.com/science/article/pii/S1934590915003173
- http://www.cell.com/cell-stem-cell/abstract/S1934-5909%2815%2900305-7
- Published in Corporate News / Blog
New Stem Cell Research Shows Promising Results for Muscular Dystrophy
The term muscular dystrophy (MD) refers to a group of disorders in which a genetic abnormality causes muscles responsible for controlling movement to become weak, and muscle mass to be lost. These inherited disorders usually affect voluntary (skeletal) muscles, although weakness can also extend to the muscles that control respiration and swallowing.
Given that the genetic mutations triggering MD interfere with the normal production of certain critical proteins, the body is not able to reverse muscle weakening or loss of mass, so even when the disease progresses slowly, it eventually affects one’s ability to walk in a more or less conducive manner.
Who is affected by muscular dystrophy?
In most cases MD appears in infancy, but it’s not uncommon for symptoms to start manifesting in teens or adults.
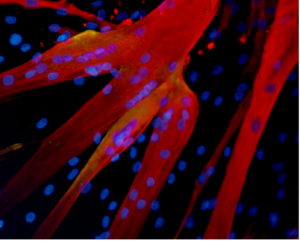
Muscle fibers formed in the lab by human mesoangioblasts (image: eurostemcell).
There are different kinds of muscular dystrophy, the most common and severe form being Duchenne muscular dystrophy (DMD) Caused by a genetic flaw or defect, Duchenne MD is more common in males than females [1} and affects about 1 in every 3,500 boys worldwide.
The onset of Duchenne muscular dystrophy occurs between the ages of 2 and 6, and evolves slowly. Muscles becoming weaker year after year, and the spine and limbs becoming progressively deformed. In most cases, children affected by this form of the disease become wheelchair dependent by the age of 12.
People suffering from Duchenne MD often die in their 20s, and those who survive usually experience some degree of cognitive impairment. The shortening of tendons and muscles limits the mobility of sufferers even more, and breathing and heart problems can occur.
Treatments for Duchenne muscular dystrophy
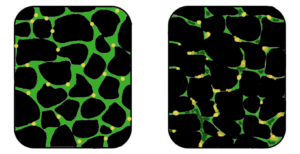
Muscular dystrophy is a genetic disorder where the muscle tissue wastes away and loses function. In the affected muscle (left), the tissue has become disorganized and the concentration of dystrophin (green), an important protein in normal muscle functioning, is greatly reduced. (Image: Wikipedia)
Physiotherapy is commonly used for slowing down the loss of muscle mass and for maintaining flexibility or reducing muscle stiffness. Steroids are also used to slow down muscle wasting, but the severe side effects of steroids often cause more harm than good, such as bone weakening or cardiovascular problems.
In a healthy organism, damaged muscles repair themselves thanks to a series of cells that include muscle stem cells, called satellite cells. In Duchene muscular dystrophy, the muscles lack dystrophin, the protein needed for maintaining the integrity of muscle fibers. Without this protein, the burden placed on the body’s naturally occurring muscle stem cells is too intense, rendering the cells unable to repair damaged muscle tissue or to generate new muscle mass to replace wasted mass [6].
For this reason, scar tissue and fat cells take the place of damaged muscle tissue, contributing to muscle weakening and, over time, cause muscles to lose their functional ability. Would it be possible for the damaged muscle fibers to regain their regenerative ability with help from transplanted stem cells?
Research suggests stem cells could be a potential solution for muscle wasting
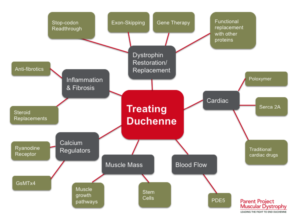
(Click on image to enlarge) Considerable efforts are underway to develop drugs and biologics (cell and gene therapy) to address the primary problem in Duchenne—the absence of dystrophin. Restoring dystrophin or replacing dystrophin with replacement protein are considered foundational therapies.
In 2006, researchers managed to restore mobility in two afflicted dogs using stem cells isolated from muscle blood vessels [4], and in 2007 scientists managed to treat Duchenne MD in research mice using a combination of genetic correction and stem cells [3]. The latter study showed that it is possible to correct the genetic error in the cells that no longer produce dystrophin protein, and inject corrected cells stimulating the regeneration of muscles.
Researchers at the Harvard Stem Cell Institute obtained similar results, demonstrating that transplanted muscle stem cells can improve function in mice with MD, while replenishing the stem cell population in muscle fibers [5].
Although it’s still too early to say whether stem cells can cure DMD in humans, it’s clear that there are some promising stem-cell-based approaches for Duchenne MD. One solution is to replace the defective stem cells with healthy stem cells, as these may be able to generate working muscle fibers to replace damaged muscle fibers .
A second solution would be to reduce the inflammation that speeds up the loss and weakening of muscles using certain types of stem cells [2]. Combined treatments, such as mixing stem cell therapies with gene therapies are also being tested and may prove successful in the near future.
References:
- http://www.mayoclinic.org/diseases-conditions/muscular-dystrophy/basics/definition/con-20021240
- http://www.eurostemcell.org/factsheet/muscular-dystrophy-how-could-stem-cells-help
- https://www.mda.org/disease/duchenne-muscular-dystrophy/research
- http://quest.mda.org/article/scientists-bullish-stem-cells-muscle-repair
- http://hsci.harvard.edu/stem-cells-used-treat-muscular-dystrophy-mice
- https://med.stanford.edu/news/all-news/2014/12/stem-cells-faulty-in-duchenne-muscular-dystrophy.html
- Published in Corporate News / Blog
A Good Night's Sleep Protects Stem Cells From Premature Aging
If you’re one of those people who is really fond of their beauty sleep, or who never compromises when it comes to getting their full eight hours per night, now you have one more reason to make a full night’s sleep a priority .
A study by scientists at the German Cancer Research Center have found that while environmental stress can damage the DNA in adult hematopoietic stem cells, a good night’s sleep can keep these cells young, contributing to a youthful appearance and preventing cancer.
Healthy sleep patterns lower the risk of DNA damage in stem cells
According to German researchers, under normal conditions a high number of different types of adult stem cells exists in a state of dormancy inside the human body, but they cannot divide, therefore cannot be used for tissue regeneration. This state of dormancy protects the stem cells from DNA damage, keeping us younger and preventing premature aging [1].
Yet, increased levels of stress in all its forms—from chronic infections to environmental stress—can trigger a rapid division of stem cells, kicked into gear as the body needs to repair its damaged tissues. In such conditions, the dormant stem cells go from no activity to very high activity in a short interval, and this rapid change forces them to increase their metabolic rate and synthesize new DNA.
Doctor Michael Milsom, who coordinated the German study, says that having to simultaneously execute such complicated functions increases the risk of DNA damage in the stem cells, reducing the ability of tissues to repair themselves and speeding up aging [1, 3].
Moreover, scientists believes that the accumulation of stress-induced damage in the stem cells can make one more prone to cancer. Experiments conducted in this study showed that cell division that takes place under stress leads to an increased production of reactive metabolites. These substances can damage DNA, causing the death of stem cells or leading to mutations that can contribute to cancer.
Understanding how to prevent the aging of stem cells or DNA mutations and damage could be the key to delaying the aging process and reducing the risk of developing certain forms of cancer, concludes Dr. Trumpp, co-author of the study’s research paper.
Protect your stem cells for healthy skin and a youthful appearance
The study is not the only one to prove a connection between sleep and the health of stem cells. Another paper, published in the journal of Cell Research by scientists from the University of California Irvine, showed that circadian rhythms regulate the metabolism of skin stem cells, and that getting enough sleep during the night can maintain healthy cell division, nurturing stem cell differentiation [2].
Although the study was conducted on mice, the findings are worth exploring further to determine whether a disruption in the healthy circadian rhythm can alter the normal function of stem cells, leading to accelerated aging.
Professors Andersen and Gratton, who conducted the Irvine study, focused on the effects stem cells have on the skin, already knowing that stem cells found in the dermal layers protect the skin and help in the repairing the epidermis after injuries.
Using innovative technologies, the two researchers measured the metabolic state of stem cells, discovering that the circadian clock does regulate one form of intermediary metabolism in target cells. According to researchers, it’s the same component of metabolism that creates oxygen radicals, harmful substances that can cause DNA damage.
The results of this study suggest that maintaining healthy sleep patterns can prevent DNA-damage in skin stem cells, while an altered internal clock could lead to the accumulation of damage in these cells, accelerating aging.
References:
[1] http://www.sciencedaily.com/releases/2015/02/150218122951.htm
[2] http://www.cell.com/cell-reports/abstract/S2211-1247%2814%2901018-3
[3] http://en.wikipedia.org/wiki/DNA_damage_theory_of_aging
- Published in Corporate News / Blog