Global Stem Cells Group announces the opening of a new Stem Cell Center in Bandung, Indonesia
Miami, FL, December 12, 2022 – Global Stem Cells Group (GSCG) is pleased to announce the opening of a new Stem Cell Center in Bandung, Indonesia in partnership with the Dr. Yanti Aesthetic Clinic. This joint venture will be the second in the country as they already have another facility in the stunning City of Surabaya.
The New Global Stem Cell Group Facility is Expected to Transform the Regenerative Medicine Field
The GSCG’s new center in Bandung aims to increase awareness about the benefits of regenerative medicine. Its launch strengthened the relationship between GSCG and the Dr. Yanti Aesthetic Clinic. This new facility is expected to transform regenerative medicine in the following ways:
- Offering accessible stem cell therapy to Indonesians;
- Promoting regenerative medicine technology; and
- Inspiring other doctors and industry experts to explore regenerative medicine.
Top Government Officials Attended the Launch of the Global Stem Cell Group Office in Bandung
The launch of the new Global Stem Cell Group facility in Bandung was a remarkable event in the medical field. The Minister of Tourism was among the top officials that witnessed the launch. Furthermore, the office of the Vice President sent several representatives.
What the President of Global Stem Cell Group Has to Say
Benito Novas, the president of the GSCG, was among the top industry specialists that graced the launch of the company’s second facility in Indonesia. According to him, GSCG wants to make regenerative medicine readily available for patients worldwide. Likewise, the company is encouraging more doctors to adopt stem cell therapy’s clinical and aesthetic applications in their work.
Benito Novas said: “We are dedicated to making it possible for both doctors and patients in all parts of the world to experience the benefits of regenerative medicines. The company is expanding and establishing itself as a market leader.”
The Director of the Dr. Yanti Aesthetic Clinic Appreciates GSCG
Dr. Yanti Kushmiran, director of the DR. Yanti Aesthetic Clinic, is once again honored to be part of this step into the future of regenerative medicine with its second Stem Cell Center in Indonesia together with the leading company Global Stem Cells Group.
Regarding this new clinic, Dr. Yanti says “We are honored to be part of GSCG, which has a more than 10-year track record in the market and a strong international reputation and to open a second cell therapy and regenerative medicine center facility under the Stem Cell Center brand. We will be able to provide more services to our patients as a result of this new partnership.”
Another GSCG Clinic is Opening in Indonesia by January 2023
The Global Stem Cell Group will continue increasing patients’ access to advanced regenerative medicine. As a result, the company plans to open another branch in Jakarta, the Capital of Indonesia. GSCG and its partners plan to launch this third facility in January 2023.
About Global Stem Cell Group
The Global Stem Cell Group is a family of several companies focused on stem cell medicine and research. The company uses its network to bring leadership in regenerative medicine training, research, and patient applications.
GSCG’s mission is to allow physicians to present the benefits of stem cell medicine to patients worldwide. The company also partners with policymakers, educators, and regulators to promote regenerative medicine.
Global Stem Cells Group is a publicly traded company operating under the symbol MSSV. https://finance.yahoo.com/quote/mssv/
To learn more about Global Stem Cells Group, Inc.’s companies visit our website www.stemcellsgroup.com or call +1 305 560 5331
- Published in Press Releases
Global Stem Cells Group, Announces Launch of New Stem Cells and Regenerative Medicine Clinic in CDMX
Miami, FL, October 28, 2022 – Global Stem Cells Group announces a new partnership that enhances its goal of establishing its therapies and technology to meet market demand in populated areas of the world.
This collaboration with STEM LIFE clinic’s new facility and Dr. Vanessa Rodriguez Pares, currently one of the most prestigious aesthetic clinics in Mexico City, is expected to promote a high level of service in regenerative medicine throughout the country.
As part of this effort, the International Society for the Application of Stem Cells (ISSCA) has granted Dr. Vanessa Rodriguez Peers affiliation and use of their brand, products, therapies and training on how to apply stem cell therapies.
“This new partnership aims to expand the Global Stem Cells Group (GSCG) brand and create centers of excellence in cell therapy to meet the high demand in the Mexican market,” said Benito Novas, CEO of Global stem cells group “GSCG is rapidly expanding its global operations as it seeks to become a major player in the lucrative regenerative medicine industry. To achieve our expansion plans, our organization is partnering with healthcare providers specializing in regenerative medicine with at least five years of experience in the healthcare sector.”
Stem cell therapy is becoming an increasingly effective clinical solution for treating conditions that traditional or conventional medicine only offers within palliative care and pain management. Patients around the world are seeking a natural regenerative alternative without the potential risks and side effects sometimes associated with conventional pharmaceuticals.
The opening of this center, which will include the construction of an autologous tissue processing laboratory and an allogeneic tissue bank, will help stem cell therapy and regenerative medicine finally move from being an elective procedure to being accessible to patients throughout Mexico.
About Dr. Vanessa Rodriguez Pares
Dr. Vanessa Rodriguez Pares is a specialist in aesthetic medicine and surgery, with special attention to obesity and overweight in all ages. Management of cosmetic surgery with a comprehensive approach to the patient, taking into account their safety and their physical, psychological and emotional needs. Specialist in ULTHERAPY treatment to perform facelift without surgery. In addition to extensive experience with cellular therapies and regenerative medicine since 2016, specializing in anti-aging techniques, hair regeneration, facial aesthetics with stem cell assisted lipotransfer among other techniques.
About the Global Stem Cell Group
Global Stem Cells Group, Inc. is the parent company of six wholly owned operating companies dedicated entirely to stem cell research, training, products, and solutions. Founded in 2012, the company combines dedicated researchers, physician and patient educators, and solution providers with the shared goal of meeting the growing worldwide need for leading edge stem cell treatments and solutions. With a singular focus on this exciting new area of medical research, Global Stem Cells Group and its subsidiaries are uniquely positioned to become global leaders in cellular medicine.
Global Stem Cells Group’s corporate mission is to make the promise of stem cell medicine a reality for patients around the world. With each of GSCG’s six operating companies focused on a separate research-based mission, the result is a global network of state-of-the-art stem cell treatments.
Global Stem Cells Group is a publicly traded company operating under the symbol MSSV. https://finance.yahoo.com/quote/mssv/
To learn more about Global Stem Cells Group, Inc.’s companies visit our website
www.stemcellsgroup.com or call +1 305 560 5331
- Published in Press Releases
ISSCA Announces the Regenerative Medicine World Congress Speaker list and Promgram
The International Society for Stem Cell Application (ISSCA) has announced the Regenerative Medicine World Congress 2022 that will take place at the Radisson Blu Hotel in Istanbul, Turkey, between September 23rd and 25th, 2022. The congress brings together world-class doctors and scientists from over 35 countries.
Participants at the congress will have a rare chance to listen to over 25 world-leading medical experts as they make evidence-based research presentations on various issues related to stem cell therapy and regenerative medicine via evidence-based presentations.
Highlights of the Congress
Interactions with World-Class Speakers
Participants at the congress will have a chance to engage scientists and physicians with extensive experience applying cellular therapies and regenerative medicine in clinical environments. It will be an opportunity to gain insights into the industry and applications of this technology.
Updates on the Latest Products and Technologies in Regenerative Medicine
Some items to be discussed at the congress include new technologies, the latest products and equipment in regenerative medicine, and cellular studies. Participants will access these technologies and understand how they play a part in developing solutions to different problems in the medical field.
Hands-on Training by Leading Instructors
Participants will get two days of intensive training on clinical and aesthetic applications of stem cell therapy as part of the workshop. This training will be provided by ISSCA-certified instructors with experience in the particular areas that they will be handling. On the third day. participants will participate in live practical lessons on areas lectured on in the last two days. However, the practical lessons will be limited to 20 seats and available only to participants who purchase the all-access package.
An Opportunity to Network
Doctors and scientists will get an opportunity to network with like-minded professionals from different parts of the world to expand their knowledge and be part of a growing team of innovative minds who are leading the future of medicine. Participants will have the opportunity to interact, share ideas, and contact fellow professionals and speakers at the congress.
The Program
The president of ISSCA. Dr. Dayeong Kim (Korea). will open the program before the rest of the instructors take the stage. This team will tackle diverse areas that show the use of stem cell therapy in various medical conditions and real-world cases where the technology is being applied. Participants will get a chance to gain insights and expand their thoughts on the matters surrounding the use of stem cell technology to deal with various medical conditions.
Conference Packages
There are two packages for the conference. The first package will give participants access to two-day lectures on September 23rd and 24th. This package is going for $499.
The second package, dubbed “All-Access.” will include the one-day hands-on certification at ReGen iC Clinic on September 25th. in addition to two two-day lectures. This package will go for $950.
There are only 20 seats in the All-Access package. You can reserve a seat in either of the packages by purchasing the ticket on the ISSCA website.
More information regarding the conference can be found on the ISSCA website.
- Published in Press Releases
Platelet-Rich Plasma For Melasma — Will It Fade Forever?
For most women, a tiny pimple on the face is enough to ruin their day. Or week. Even the slightest imperfection that may have a 1% chance of getting noticed by others will freak them out. For these women, Melasma is their darkest nightmare. It’s a pretty common issue, a result of exposure to sun, that causes brown patches on the face. Permanent patches, I should add.
If you’re suffering from Melasma, the road to “recovery” usually looks like this.
- You hope that it’ll fade away.
- Your friend suggests you try apple cider vinegar and lemon juice treatment.
- Slightly disappointed.
- You visit a dermatologist who’ll prescribe a bleaching cream (hydroquinone or similar).
- Full-on disappointment.
- You Google the hell out of the topic.
- Overwhelm.
- Concealers and makeup becomes your best friend.
At this point, no one can convince you there is a treatment for getting rid of melasma. Trying more and more treatment only runs the risk of making the condition worse. So what would you do?
Platelet-Rich Plasma For Melasma
What about Platelet-Rich Plasma For Melasma?
According to recent Turkish and Malaysian studies, Platelet-Rich Plasma is showing great promise for melasma. The one good thing about PRP for Melasma is the fact that PRP won’t make the condition worse unlike IPL, fraxel or other treatments. So that’s one of the treatment you can confidently try without worry. It’s like getting a natural facial treatment that has a whole lot of potential benefits even if it didn’t help cure melasma.
PRP injections work by supplying growth factors to reduce the pigmentation. And being an independant treatment with no downtime, it can be done in conjunction with conventional treatments for melasma to add and enhance the effects. There are more than 30 bioactive substances in Platelet-Rich Plasma that has separate roles like increasing skin volume and adding new blood vessels to name a few.
Platelet-Rich Plasma with Microneedling
This is the most common combination for Platelet-Rich Plasma therapy. Here’s a video of Dr. Michael Somenek performing PRP injection on a patient of his immediately after microneedling. The combination is known to have produced results for a lot of varieties of skin pigmentation issues that it’d not be wise for anyone to ignore it for melasma, especially when creams and peels didn’t help. More important is PRP’s ability to stimulate collagen production in the area so it tightens the pores and makes your skin glowing.
Why Platelet-Rich Plasma?
PRP is primarily a healing vehicle. It needs to be injected into the membrane below the skin. The way it works is by supplying the underlying skin membrane with collagen and tenascin stimulated by the transforming growth factors in PRP. These growth factors also promote formation of new blood vessels that in some cases results in disappearance of spider veins.
The released growth factors (mainly platelet derived growth factor (PDGF), epidermal growth factor (EGF), vascular endothelial growth factor (VEGF), fibroblast growth factor (FGF) and transforming growth factor-beta (TGF-ß)) can stimulate proliferation of fibroblast and epidermal cell, and collagen synthesis. In addition, the transforming growth factor-beta (TGF-ß) has been proven to inhibit melanogenesis — or reverse skin pigmentation — the exact opposite effect of exposure to UV-B radiation.
Typically, patients see excellent results with 2-3 PRP injections in the first 3 months. And clinical studies have shown that it will maintain after 6 months.
However, Melasma is known to recur even after successful treatments. So you must take precautions against it by using sunscreen with broad-spectrum protection and an SPF of 30 or higher. And avoid skin care products that are harsh as they can exacerbate melasma.
- Published in Corporate News / Blog
Insulin-producing Stem Cells Grown in the Lab Mark a New Era in Stem Cell Therapies for Diabetes
A new discovery by researchers on how to activate lab-grown beta cells to mature into functioning cells that produce and release insulin in response to glucose take a significant step toward a cell therapy treatment for diabetes.
Difficulties in manipulating beta cells derived from human stem cells to mature beyond the precursor stage into fully functioning insulin releasers has been an on-going challenge for researchers..
However, researchers from the Salk Institute for Biological Studies and a team of researchers have achieved this goal with lab-grown beta cells by activating a protein called estrogen-related receptor γ (ERRγ). Their study findings were recently published in the journal Cell Metabolism.
Self-renewing capacity of human pluripotent stem cells (hPSCs)
Ronald Evans, senior author of the study, titled, “ERRγ Is required for the Metabolic Maturation of Therapeutically Functional Glucose-Responsive β Cells,”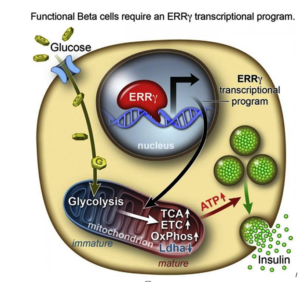 says the self-renewing capacity of human pluripotent stem cells (hPSCs) and their ability to differentiate into most cell types—from neurons to skin cells, to muscles cells and insulin-producing pancreatic beta cells—has inspired many research teams to find ways to make glucose-responsive beta cells in the lab.
says the self-renewing capacity of human pluripotent stem cells (hPSCs) and their ability to differentiate into most cell types—from neurons to skin cells, to muscles cells and insulin-producing pancreatic beta cells—has inspired many research teams to find ways to make glucose-responsive beta cells in the lab.
Evans and his research team discovered the answer to the insulin-releasing cell conundrum, and summed it up thusly:
“In a dish, with this one switch, it’s possible to produce a functional human beta cell that’s responding almost as well as the natural thing.”
Evans, a molecular biologist at the Salk Institute, says that to create the different types of cells in the lab, researchers coax the pluripotent stem cells (hPSCs) down the various branching paths that fetal cells normally travel in order to differentiate into the various cell types. However, he explains there are many developmental points in this process, and in the case of lab-grown pancreatic beta cells, research kept getting stuck at an early stage.
Adult beta cells have more ERRγ protein for a very energy-intensive process
In order to determine what might trigger the next step in getting the cells to mature, the researchers compared transcriptomes of adult and fetal beta cells. The transcriptome contains, among other things, the full catalog of molecules that switch genes on and off in the genome, which led them to discover that the nuclear receptor protein ERRγ was more abundant in adult beta cells. The team was already familiar with the protein’s role in muscle cells and had studied its ability to enhance endurance running.
Evans says that in muscles, protein promotes greater growth of mitochondria—the power generators inside cells that accelerate the burning of sugars and fats to make energy.
“It was a little bit of a surprise to see that beta cells produce a high level of this regulator,” Evans says. “But beta cells have to release massive amounts of insulin quickly to control sugar levels. It’s a very energy-intensive process.”
The research team then decided to run some tests to look more closely at what role ERRγ might play in insulin-producing beta cells.
A new era in creating functional, insulin-producing beta cells
 After they genetically engineering a deficiency of ERRy in mice, the researchers found the animals’ beta cells did not produce insulin in response to spikes in blood sugar.
After they genetically engineering a deficiency of ERRy in mice, the researchers found the animals’ beta cells did not produce insulin in response to spikes in blood sugar.
Next they tried to get beta cells made from hPSCs to produce more ERRγ, and it worked! The cells in culture began to respond to glucose and release insulin.
Finally, the team transplanted the lab-grown insulin-producing beta cells into diabetic mice and found that from day one, the cells produced insulin in response to glucose spikes in the animals’ blood.
Evans and the research team were justifiably excited by the results. It appears that just switching on the ERRγ protein is sufficient to get the lab-grown beta cells to mature and produce insulin in response to glucose – both in cultures and in live animals.
Speculating on the implications of their findings, Evans suggests that when a fetus is developing, because it gets a steady supply of glucose from the mother, it does not need to produce insulin to regulate its blood sugar, so the switch is inactive. But, when the baby is born and takes its first breath and takes in oxygen, this activates the switch.
Previous lab attempts to produce beta cells got stuck at the fetal stage. The Salk Institute researchers discovered how to take it to the adult stage, using the same protein that is switched on in nature.
“I believe this work transitions us to a new era in creating functional beta cells at will,” Evans says.
He and his research team now plan to examine how the switch might work in more complex models of diabetes treatments.
The Salk Institute study proceeds another study Medical News Today in which researchers generated mini-stomachs that produce insulin when transplanted into mice.
###
- Published in Corporate News / Blog




![melasma[1]](https://stemcellcenter.net/wp-content/uploads/2019/11/melasma1-460x260_c.jpg)