Stem Cell Research and Stem Cell Therapy: When can stem cells be used to treat patients?
The difference between stem cell research and therapy is in the scientific evidence that supports therapeutic intervention to be beneficial for the patient.
Stem cells have the remarkable potential to develop into many different types of cells in the body during early life and growth. In addition, in many tissues, stem cells serve as a sort of internal repair system, dividing essentially without limit to replenish other cells as long as the individual is alive. When a stem cell divides, each new cell has the potential either to remain a stem cell or become another type of cell with a more specialized function, such as a muscle cell, a red blood cell, or a brain cell.
Stem cell research on adult stem cells
Stem cells are distinguished from other cell types by two important characteristics. First, they are unspecialized cells capable of renewing themselves through cell division, sometimes after long periods of inactivity. Second, under certain physiologic or experimental conditions, they can be induced to become tissue- or organ-specific cells with special functions. In some organs, such as the gut and bone marrow, stem cells regularly divide to repair and replace worn out or damaged tissues. In other organs, such as the pancreas and the heart, stem cells only divide under special conditions.
Until recently, scientists primarily worked with two kinds of stem cells from animals and humans: embryonic stem cells and non-embryonic “somatic” or “adult” stem cells in stem cell research.
In 2006, researchers made a breakthrough by identifying conditions that would allow some specialized adult cells to be “reprogrammed” genetically to assume a stem cell-like state. This new type of stem cell is called induced pluripotent stem cells (iPSCs).
Stem cells are important for living organisms for many reasons. In the 3- to 5-day-old embryo, called a blastocyst, the inner cells give rise to the entire body of the organism, including all of the many specialized cell types and organs such as the heart, lungs, skin, sperm, eggs and other tissues. In some adult tissues, such as bone marrow, muscle, and brain, discrete populations of adult stem cells generate replacements for cells that are lost through normal wear and tear, injury, or disease.
Stem cell research for treating disease
Given their unique regenerative abilities, stem cells offer new potentials for treating diseases such as diabetes, and heart disease. However, much work remains to be done in the laboratory and the clinic to understand how to use these cells for cell-based therapies to treat disease, which is also referred to as regenerative or reparative medicine.
Laboratory studies of stem cells enable scientists to learn about the cells’ essential properties and what makes them different from specialized cell types. Scientists are already using stem cells in the laboratory to screen new drugs and to develop model systems to study normal growth and identify the causes of birth defects.
Research on stem cells continues to advance knowledge about how an organism develops from a single cell and how healthy cells replace damaged cells in adult organisms. Stem cell research is one of the most fascinating areas of contemporary biology, but, as with many expanding fields of scientific inquiry, research on stem cells raises scientific questions as rapidly as it generates new discoveries.
In 1964, the World Medical Association developed the Declaration of Helsinki as a statement of
ethical principles for medical research involving human subjects. It includes research on identifiable human material and data, last amended in October 2013.
According to the Helsinki Declaration, in the treatment of an individual patient where proven interventions do not exist or other known interventions have been ineffective, the physician, after seeking expert advice, with informed consent from the patient or a legally authorized representative, may use an unproven intervention if in the physician’s judgement it offers hope of saving life, re-establishing health or alleviating suffering.
Intervention should subsequently be made the object of research, designed to evaluate its safety and efficacy. In all cases, new information must be recorded and, where appropriate, made publicly available.
- Published in Corporate News / Blog
Global Stem Cells Group Advisory Board Member Duncan Ross, Ph.D., to Speak at Santiago, Chile Symposium
Global Stem Cells Group Advisory Board member Duncan Ross, Ph.D., founder of Kimera Research Labs, will be the keynote speaker at the Global Stem Cells Group Symposium in Santiago, Chile July 1-2, 2016.
 MIAMI, April 26, 2016–Global Stem Cells Group has announced that affiliate Kimera Research Labs founder Duncan Ross, Ph.D., a GSCG Advisory Board member, will be the keynote speaker at the Asia-Pacific Symposium in Santiago Chile, July 1-2, 2016. The abstract for Ross’s lecture will be, “The mechanism of action of stem cells in regenerative medicine is increasingly being understood to be effected through paracrine factors. Central to the question of when and how to treat an individual disease is where and for what duration a transplanted cell will persist to generate these factors.”
MIAMI, April 26, 2016–Global Stem Cells Group has announced that affiliate Kimera Research Labs founder Duncan Ross, Ph.D., a GSCG Advisory Board member, will be the keynote speaker at the Asia-Pacific Symposium in Santiago Chile, July 1-2, 2016. The abstract for Ross’s lecture will be, “The mechanism of action of stem cells in regenerative medicine is increasingly being understood to be effected through paracrine factors. Central to the question of when and how to treat an individual disease is where and for what duration a transplanted cell will persist to generate these factors.”
In the absence of a robust ability to track cell persistence in humans, Dr. Ross will present current research in murine hematopoietic and mesenchymal stem cell transplantation with support from human transplant results.

Duncan Ross, Ph.D.
The symposium will be co-sponsored by Global Stem Cells Group and the University of Santiago’s Biochemistry and Molecular Biology Department, and will focus on regenerative medicine and stem cell applications to anti-aging and aesthetic medicine. University of Santiago faculty will lead the symposium, which will host qualified academic and medical groups from around the world who will present their scientific papers.
The symposium is the first joint endeavor between Global Stem Cells Group and the University of Santiago since establishing an alliance recently, and which will be announced at the Asia-Pacific Symposium. It also marks Ross’s first appearance as a member of the Global Stem Cells Group Advisory Board.
Ross received a Ph.D. in Immunology from the University of Miami and specializes in research, mesenchymal stem cell applications, hematopoietic stem cell transplantation for hematologic disorders, the suppression of graft vs. host disease, and var
ious methods of immune suppression.
Global Stem Cells Group and Kimera Labs share a commitment to research and development, and providing stem cell treatments to patients in clinical settings worldwide.
To learn more, visit the Global Stem Cells Group website, email bnovas(at)stemcellsgroup(dot)com, or call +1 305 560 5337.
About Global Stem Cell Group:
Global Stem Cells Group, Inc. is the parent company of six wholly owned operating companies dedicated entirely to stem cell research, training, products and solutions. Founded in 2012, the company combines dedicated researchers, physician and patient educators and solution providers with the shared goal of meeting the growing worldwide need for leading edge stem cell treatments and solutions. With a singular focus on this exciting new area of medical research, Global Stem Cells Group and its subsidiaries are uniquely positioned to become global leaders in cellular medicine.
Global Stem Cells Group’s corporate mission is to make the promise of stem cell medicine a reality for patients around the world. With each of GSCG’s six operating companies focused on a separate research-based mission, the result is a global network of state-of-the-art stem cell treatments.
About Kimera Labs:
Kimera Labs is currently focused on the use of mesenchymal stem cells (MSCs) for the suppression of various immune mediated pathologies and regenerative medicine in the US, Latin America, and the Bahamas. Founder Duncan Ross, Ph.D., is an immunologist and researcher who has studied hematopoietic stem cell transplantation for hematologic disorders, the suppression of graft vs. host disease, and various methods of immune suppression.
Kimera Labs provides patients access to stem cell treatment in the U.S. according to U.S. laws. In order to provide the greatest benefit to patients, Ross frequently travels to treat patients in Central and South America where specialists are available in a different regulatory environment.
###
To view this press release online, click here
- Published in Press Releases
Global Stem Cells Group and Kimera Labs Announce Autologous Stem Cell Research Alliance
Global Stem Cells Group and Kimera Research Labs have announced an alliance to conduct scientific research on highly manipulated cells and culture expansion, and cryopreservation of autologous stem cells.
MIAMI, April 26, 2016–Global Stem Cells Group and Kimera Research Labs have announced an alliance to conduct  scientific research on highly manipulated stem cells and culture expansion, and cryopreservation of autologous stem cells. The collaboration will open new opportunities for GSCG to increase its participation in scientific research and development of new stem cell protocols and treatments for a number of conditions.
scientific research on highly manipulated stem cells and culture expansion, and cryopreservation of autologous stem cells. The collaboration will open new opportunities for GSCG to increase its participation in scientific research and development of new stem cell protocols and treatments for a number of conditions.
The manipulation of stem cells involves the ability to deliver molecules into adherent cells without disrupting differentiation, a process biotechnology researchers need in order to advance both fundamental knowledge and the state-of-the-art in stem cell research. Differentiation is the process by which an unspecialized cell, such as a stem cell, becomes specialized into one of the many cells in the body. During differentiation, certain genes become activated and other genes become inactivated in an painstakingly regulated manner. As a result, a differentiated cell develops specific structures and performs certain functions that ultimately allows it to replace damaged or dead cells. In the laboratory, a stem cell can be manipulated to become specialized or partially specialized cell types, such as heart muscle, nerve, or pancreatic cells.
“Non-destructive manipulation of stem cells in the correct environment is key to enabling technology needed within the biology and medical research communities,” says Benito Novas, CEO of Global Stem Cells Group. “To realize the promise of stem cell-based therapies to treat injuries and diseases, scientists must be able to manipulate stem cells so that they possess the necessary characteristics for successful differentiation, transplantation, and engraftment.”
To bring successful new treatments to the clinic, scientists need to control certain steps for stem cells to be useful for transplant purposes. Researchers are constantly discovering new ways to manipulate stem cells to be reproducibly made to:
- Replicate extensively and generate sufficient quantities of cells for making tissue.
- Differentiate into the desired cell type(s).
- Survive in the recipient after transplant.
- Integrate into the surrounding tissue after transplant.
- Function appropriately for the duration of the recipient’s life.
- Avoid harming the recipient in any way.
Scientists are also experimenting with different research strategies to generate tissue without the concern of immune rejection.
 Research on cryopreservation of autologous stem cells is necessary for cell bank procedures in which stem cell expansion and use are not immediately needed. Cryopreservation allows for the long-term storage of hematopoietic stem cells (HSCs) and is the preferred storage technique for virtually all components intended for autologous HSC transplantation.
Research on cryopreservation of autologous stem cells is necessary for cell bank procedures in which stem cell expansion and use are not immediately needed. Cryopreservation allows for the long-term storage of hematopoietic stem cells (HSCs) and is the preferred storage technique for virtually all components intended for autologous HSC transplantation.
Cryopreservation allows the administration of multiple-day transplant conditioning regimens as well as elective storage for patients to receive transplants at a subsequent point in a course of treatment, and offers patients the opportunity to benefit from multidose protocols.
Global Stem Cells Group and Kimera Labs share a commitment to research, develop and provide stem cell treatments to patients worldwide in a clinical setting.
To learn more, visit the Global Stem Cells Group website, email bnovas(at)stemcellsgroup(dot)com, or call +1 305 560 5337.
About Global Stem Cell Group:
Global Stem Cells Group, Inc. is the parent company of six wholly owned operating companies dedicated entirely to stem cell research, training, products and solutions. Founded in 2012, the company combines dedicated researchers, physician and patient educators and solution providers with the shared goal of meeting the growing worldwide need for leading edge stem cell treatments and solutions. With a singular focus on this exciting new area of medical research, Global Stem Cells Group and its subsidiaries are uniquely positioned to become global leaders in cellular medicine.
Global Stem Cells Group’s corporate mission is to make the promise of stem cell medicine a reality for patients around the world. With each of GSCG’s six operating companies focused on a separate research-based mission, the result is a global network of state-of-the-art stem cell treatments.
About Kimera Labs:
Kimera Labs is currently focused on the use of mesenchymal stem cells (MSCs) for the suppression of various immune mediated pathologies and regenerative medicine in the US, Latin America, and the Bahamas. Founder Duncan Ross, Ph.D., is an immunologist and researcher who has studied hematopoietic stem cell transplantation for hematologic disorders, the suppression of graft vs. host disease, and various methods of immune suppression.
Kimera Labs provides patients access to stem cell treatment in the U.S. according to U.S. laws. In order to provide the greatest benefit to patients, Ross frequently travels to treat patients in Central and South America where specialists are available in a different regulatory environment.
###
To view this press release live on line, click here
- Published in Press Releases
German Stem Cell Scientists Develop 3-D “Mini-retinas” –New Hope for Restoring Sight in Patients with Retinal Degeneration Caused by Diabetes and Inherited Disorders.
Medical breakthroughs using stem cells are aimed at all parts of the body bones, kidneys, joints, spines–and now, sight.
A German study in March in Stem Cell Reports, reports that scientists have created an efficient way of developing 3D retina organoids leverage the self-organizing properties of stem cells to create diverse multi-cellular tissue proxies.
3-D Mini-retinas protocol
The new mini-retina protocol involves cutting an organoid grown from stem cells into three, half-moon shaped pieces at an early stage of eye development. Each of these pieces eventually grows into the full suite of cells found in the retina.
3-D retinal organoids developed in this process efficiently replicate retina formation. This includes the light-detecting cone cells, which now can be produced in high quantities.
cone cells, which now can be produced in high quantities.
Cone photoreceptors, which are responsible for high acuity and color vision, are the most precious retinal cell type with regard to potential future cell replacement therapies in patients affected by retinal degeneration caused by diabetes and inherited disorders.
The process of developing 3D retinal organoids also allows the surviving organoids to grow to reach sizes similar to uncut organoids. These mini-retinas swim around in the dish and because they’re not attached to a surface, better reflect the structure of retinal tissue during development.
In the past, the inability to produce such cells has been a major limitation for regenerative medicine; however, this new method increases the yield of retinal organoids 4-fold, allowing researchers to take a great step forward in the study of the retina and how to repair it.
3-D Mini-retinas offer more diverse ways to study retina tissue
 “The goal isn’t just to make the closest thing next to a real retina, but also to possibly harness the flexibility of the system to create more diverse ways of studying retina tissue,” says senior author Mike Karl, of the German Center for Neurodegenerative Diseases (DZNE) and part of the Center for Regenerative Therapies (CRTD) at Technische Universität Dresden.
“The goal isn’t just to make the closest thing next to a real retina, but also to possibly harness the flexibility of the system to create more diverse ways of studying retina tissue,” says senior author Mike Karl, of the German Center for Neurodegenerative Diseases (DZNE) and part of the Center for Regenerative Therapies (CRTD) at Technische Universität Dresden.
“Even with our new additions to existing organoid systems, we have not yet reached that tipping point of robustness that we need for people without the expertise to grow these models.”
Karl and his colleagues’ comparative studies on pluripotent stem cell-derived human and mouse retina organoids and mouse retina in vivo support the power of the new organoid protocol.
New insights in the study of retinal disease
“Tissue heterogeneity (diversity) is a major challenge in organoid systems, and here our work provides new insight, which will help to develop specific organoid-based models, specifically to reliably study retinal disease mechanism,” says Karl.
The Karl Lab’s change to the mini-retina protocol involves cutting a retina organoid grown from stem cells into three pieces at an early stage of eye development. Each of these pieces, which look like little half moons, eventually grows into the full suite of cells found in the retina, thereby increasing the yield of retinal organoids up to 4-fold compared to previous protocols. Karl’s next objective is to make his 3-D “mini-retinas” even more complex, perhaps by bringing in blood vessels and using the organoids to study regeneration and the function of different neural cell types–specifically, from the human retina.
- Published in Corporate News / Blog
New Stem Cell Research Shows Promising Results for Muscular Dystrophy
The term muscular dystrophy (MD) refers to a group of disorders in which a genetic abnormality causes muscles responsible for controlling movement to become weak, and muscle mass to be lost. These inherited disorders usually affect voluntary (skeletal) muscles, although weakness can also extend to the muscles that control respiration and swallowing.
Given that the genetic mutations triggering MD interfere with the normal production of certain critical proteins, the body is not able to reverse muscle weakening or loss of mass, so even when the disease progresses slowly, it eventually affects one’s ability to walk in a more or less conducive manner.
Who is affected by muscular dystrophy?
In most cases MD appears in infancy, but it’s not uncommon for symptoms to start manifesting in teens or adults.
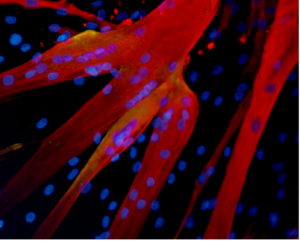
Muscle fibers formed in the lab by human mesoangioblasts (image: eurostemcell).
There are different kinds of muscular dystrophy, the most common and severe form being Duchenne muscular dystrophy (DMD) Caused by a genetic flaw or defect, Duchenne MD is more common in males than females [1} and affects about 1 in every 3,500 boys worldwide.
The onset of Duchenne muscular dystrophy occurs between the ages of 2 and 6, and evolves slowly. Muscles becoming weaker year after year, and the spine and limbs becoming progressively deformed. In most cases, children affected by this form of the disease become wheelchair dependent by the age of 12.
People suffering from Duchenne MD often die in their 20s, and those who survive usually experience some degree of cognitive impairment. The shortening of tendons and muscles limits the mobility of sufferers even more, and breathing and heart problems can occur.
Treatments for Duchenne muscular dystrophy
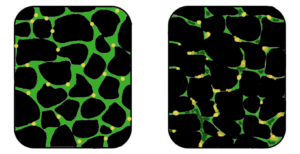
Muscular dystrophy is a genetic disorder where the muscle tissue wastes away and loses function. In the affected muscle (left), the tissue has become disorganized and the concentration of dystrophin (green), an important protein in normal muscle functioning, is greatly reduced. (Image: Wikipedia)
Physiotherapy is commonly used for slowing down the loss of muscle mass and for maintaining flexibility or reducing muscle stiffness. Steroids are also used to slow down muscle wasting, but the severe side effects of steroids often cause more harm than good, such as bone weakening or cardiovascular problems.
In a healthy organism, damaged muscles repair themselves thanks to a series of cells that include muscle stem cells, called satellite cells. In Duchene muscular dystrophy, the muscles lack dystrophin, the protein needed for maintaining the integrity of muscle fibers. Without this protein, the burden placed on the body’s naturally occurring muscle stem cells is too intense, rendering the cells unable to repair damaged muscle tissue or to generate new muscle mass to replace wasted mass [6].
For this reason, scar tissue and fat cells take the place of damaged muscle tissue, contributing to muscle weakening and, over time, cause muscles to lose their functional ability. Would it be possible for the damaged muscle fibers to regain their regenerative ability with help from transplanted stem cells?
Research suggests stem cells could be a potential solution for muscle wasting
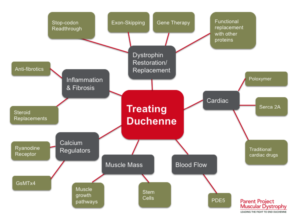
(Click on image to enlarge) Considerable efforts are underway to develop drugs and biologics (cell and gene therapy) to address the primary problem in Duchenne—the absence of dystrophin. Restoring dystrophin or replacing dystrophin with replacement protein are considered foundational therapies.
In 2006, researchers managed to restore mobility in two afflicted dogs using stem cells isolated from muscle blood vessels [4], and in 2007 scientists managed to treat Duchenne MD in research mice using a combination of genetic correction and stem cells [3]. The latter study showed that it is possible to correct the genetic error in the cells that no longer produce dystrophin protein, and inject corrected cells stimulating the regeneration of muscles.
Researchers at the Harvard Stem Cell Institute obtained similar results, demonstrating that transplanted muscle stem cells can improve function in mice with MD, while replenishing the stem cell population in muscle fibers [5].
Although it’s still too early to say whether stem cells can cure DMD in humans, it’s clear that there are some promising stem-cell-based approaches for Duchenne MD. One solution is to replace the defective stem cells with healthy stem cells, as these may be able to generate working muscle fibers to replace damaged muscle fibers .
A second solution would be to reduce the inflammation that speeds up the loss and weakening of muscles using certain types of stem cells [2]. Combined treatments, such as mixing stem cell therapies with gene therapies are also being tested and may prove successful in the near future.
References:
- http://www.mayoclinic.org/diseases-conditions/muscular-dystrophy/basics/definition/con-20021240
- http://www.eurostemcell.org/factsheet/muscular-dystrophy-how-could-stem-cells-help
- https://www.mda.org/disease/duchenne-muscular-dystrophy/research
- http://quest.mda.org/article/scientists-bullish-stem-cells-muscle-repair
- http://hsci.harvard.edu/stem-cells-used-treat-muscular-dystrophy-mice
- https://med.stanford.edu/news/all-news/2014/12/stem-cells-faulty-in-duchenne-muscular-dystrophy.html
- Published in Corporate News / Blog
- 1
- 2