MIAMI, Feb 19, 2018—Amine Rafik, M.D., Ph.D., a member of the Global Stem Cells Group Advisory Board, will be a keynote speaker at the International Association for Stem Cell Application (ISSCA) Regenerative Medicine Cell Therapies Symposium in Istanbul, Turkey April 28, 2018. Rafik will speak on the role of adipose-derived stem cells in regenerative medicine as an attractive alternative to the use of stem cells derived from bone marrow and other sources, due to their abundance in fat and their ability to be easily harvested.

Amine Rafik, M.D., Ph.D
Rafik’s lecture will include patient demographic data and digital photographs taken on the day of each patient’s surgery and every day thereafter. ASC patients received three treatments of 20 cc of autologous adipose stem cell injections in the subcutaneous tissue surrounding the ulcer with time to wound closure defined as the time at which the wound bed was completely re-epithelialized and filled with new tissue.
He will discuss study results revealing the average time for wound closure in adipose stem cell (ASC) recipient patients compared to control group patients. He will also examine the study’s conclusion that suggests local transplantation of autologous adipose stem cells could accelerate wound healing, and that some clinical aspects of wound healing, as well as the potential for therapies based on stem cells, represent a feasible therapeutic approach to the treatment of clinical wounds.
Rafik is a consultant and plastic, reconstructive and aesthetic surgeon at Al Farabi Hospital in Morocco. He specializes in reconstructive plastic surgery particularly for the face, head, and neck, as well as for patients who require reconstructive surgery following treatment for skin cancer or trauma. Rafik also performs cosmetic surgery and microsurgery.
He completed his plastic surgery training in Casablanca and was awarded full membership to the American Academy of Cosmetic Surgery in 2015, and the European Association of Maxilla-Facial surgery in 2016.
He is a reviewer for many international indexed journals, including; OncoTargets and Therapy Journal, the British Journal of Medicine and Medical Research, and the Cancer and Clinical Oncology Journal, published by Canadian Center of Science and Education, where he is a member of the editorial board.
The Istanbul international symposium is part of ISSCA’s mission to support a paradigm shift from traditional healthcare solutions to regenerative medicine and provide the latest innovative discoveries and developments in all areas of stem cell research. The symposium will host a group of renowned international speakers, experts in the field of stem cell and regenerative medicine, who will provide a full day of rigorous scientific discourse directed to physicians.
The day’s events will incorporate information on stem cell biology, medicine, applications, regulations, product development, and commercialization, business opportunities, challenges, and potential strategies for overcoming those challenges.
To participate in the ISSCA Istanbul Symposium, reserve your spot by registering today.
For more information, visit the stemcellconference.org website, email info@stemcellsgroup.com, or call +1305 560 5337.
About International Society for Stem Cell Application (ISSCA):
The International Society for Stem Cell Application (ISSCA) is a multidisciplinary community of scientists and physicians, all of whom aspire to treat diseases and lessen human suffering through advances in science, technology and the practice of regenerative medicine. ISSCA serves its members through advancements made to the specialty of regenerative medicine.
The ISSCA’s vision is to take a leadership position in promoting excellence and setting standards in the regenerative medicine fields of publication, research, education, training, and certification.
As a medical specialty, regenerative medicine standards and certifications are essential, which is why ISSCA offers certification training in cities all over the world. The goal is to encourage more physicians to practice regenerative medicine and make it available to benefit patients both nationally and globally. Incorporated under the Republic of Korea as a non-profit entity, the ISSCA is focused on promoting excellence and standards in the field of regenerative medicine.
###
ISSCA conference speaker Amine Rafik
- Published in Press Releases
ISSCA Announces Regenerative Medicine Cell Therapies Symposium in Istanbul, Turkey April 28, 2018
(Image: Istanbul, Turkey)
The International Association for Stem Cell Application (ISSCA) has announced plans to host a regenerative medicine symposium in Istanbul, Turkey April 28, 2018.
MIAMI, Feb 14, 2018—The International Association for Stem Cell Application (ISSCA) has announced plans to host a regenerative medicine symposium in Istanbul, Turkey April 28, 2018. The symposium will focus on new advances in cell therapies.
The Istanbul international symposium is part of ISSCA’s mission to support a shift from traditional healthcare solutions to regenerative medicine and provide the latest innovative discoveries and developments in all areas of stem cell research. The symposium will host a group of renowned international speakers, experts in the field of stem cell and regenerative medicine, who will provide a full day of rigorous scientific discourse directed to physicians.
The day’s events will incorporate information on stem cell biology, medicine, applications, regulations, product development, and commercialization, business opportunities, challenges, and potential strategies for overcoming those challenges.
To learn more about the ISSCA symposium in Istanbul and to register to attend, visit the stemcellconference.org website, email info@stemcellsgroup.com, or call +1305 560 5337.
About International Society for Stem Cells Applications
 The International Society for Stem Cell Application (ISSCA) is a multidisciplinary community of scientists and physicians, all of whom aspire to treat diseases and lessen human suffering through advances in science, technology and the practice of regenerative medicine. ISSCA serves its members through advancements made to the specialty of regenerative medicine.
The International Society for Stem Cell Application (ISSCA) is a multidisciplinary community of scientists and physicians, all of whom aspire to treat diseases and lessen human suffering through advances in science, technology and the practice of regenerative medicine. ISSCA serves its members through advancements made to the specialty of regenerative medicine.
The ISSCA’s vision is to take a leadership position in promoting excellence and setting standards in the regenerative medicine fields of publication, research, education, training, and certification.
As a medical specialty, regenerative medicine standards and certifications are essential, which is why ISSCA offers certification training in cities all over the world. The goal is to encourage more physicians to practice regenerative medicine and make it available to benefit patients both nationally and globally. Incorporated under the Republic of Korea as a non-profit entity, the ISSCA is focused on promoting excellence and standards in the field of regenerative medicine.
Regenerative Medicine symposium Istanbul
- Published in Press Releases
Global Stem Cells Group to Launch Regenerative Medicine Training Program for Mexico at Congreso Internacional de Medicina Antienvejecimiento y Longevidad
(Image: Regenerative Medicine Training Program for Mexico)
MIAMI, Feb. 14, 2018— Global Stem Cells Group, a world leader in stem cell and regenerative medicine, will launch the organization’s complete Regenerative Medicine Training Program for Mexico at the IX Congreso Internacional de Medicina Antienvejecimiento y Longevidad (Congress in Anti-Aging and Longevity Medicine) to be held February 16 – 18, 2018 in Mexico City.
The training course will be held in Mexico City April 13 – 14, 2018.
The “Practical Course of Cell Therapies in a Clinical Environment” is a two-day, hands-on training course that provides physician attendees with the intellectual property of 22 proprietary protocols that allow treatment procedures for degenerative and aesthetic conditions and diseases in-office, as well as step-by-step videos of each protocol.
Course attendees will acquire the skills that will allow them to offer alternative therapies to patients who suffer from degenerative conditions for which no traditional medical solutions are available. Attendees will treat three to five live patients during the course. Upon completing the course successfully, participants will join a select group of physicians at the forefront of medical science—only 5 percent of physicians worldwide have access to studies of cell therapies, and only 0.01 percent are currently practicing these therapies.
Qualified physicians learn skills that can be used to treat patients in-practice, and utilize the training for career advancement.
Participating physicians will also receive access to the online stem cell training course to review all content and procedures introduced in the 2-day clinical training course, patient forms and guidelines, procedure informed consent forms, didactic lectures, training booklets, and more.
The Regenerative Medicine Training Program for Mexico was developed for physicians and high-level practitioners to learn techniques in harvesting and reintegrating stem cells. Stem cell therapies continue to revolutionize the healthcare industry and help improve the quality of life for patients.
To learn more about the certification training course and register to participate, visit the Stem Cell Training Course website, email info@stemcellsgroup.com, or call 305-560-5337.
About Global Stem Cells Group
 Global Stem Cells Group (GSCG) is a worldwide network that combines seven major medical corporations, each focused on furthering scientific and technological advancements to lead cutting-edge stem cell development, treatments, and training. The united efforts of GSCG’s affiliate companies provide medical practitioners with a one-stop hub for stem cell solutions that adhere to the highest medical standards.
Global Stem Cells Group (GSCG) is a worldwide network that combines seven major medical corporations, each focused on furthering scientific and technological advancements to lead cutting-edge stem cell development, treatments, and training. The united efforts of GSCG’s affiliate companies provide medical practitioners with a one-stop hub for stem cell solutions that adhere to the highest medical standards.
Global stem cell’s mission is to be the largest recognized stem cell and regenerative medicine network in the world.
Regenerative Medicine Training for Mexico
- Published in Press Releases
Global Stem Cells Group CEO Benito Novas to be a Keynote Speaker at Congreso Internacional de Medicina Antienvejecimiento y Longevidad Feb. 16-18, 2018
Benito Novas, Global Stem Cells Group CEO
MIAMI, Feb. 14, 2018— Global Stem Cells Group founder and CEO Benito Novas will be a keynote speaker at the IX Congreso Internacional de Medicina Antienvejecimiento y Longevidad (Congress in Anti-Aging and Longevity Medicine) February 16 – 18, 2018 in Mexico City. The conference is organized by The Institute of Anti-Aging and Longevity Medicine, the leading anti-aging and regenerative medicine society in Mexico.
Novas, a global entrepreneur and medical marketing professional in the fields of biotechnology, life sciences, and healthcare development, will share his expertise on the latest marketing tools for managing a medical practice. Topics will include social media strategies and tools for promoting physician practices, and how to design Google and Facebook ads that generate targeted leads and engage new potential patients.
The healthcare industry is continuously pursuing new and improved medical and technological advancements, treatments, and expanded healthcare opportunities that offer a higher level of patient care. The industry serves a uniquely broad audience, and today’s consumers are searching online for medical providers.
In fact, the majority of consumers rely on the internet to find healthcare providers.
For this reason, Novas says it is critical for medical practices and providers to stay on top of digital marketing strategies and trends in order to keep their organizations competitive and to generate new leads in today’s patient-centric market.
Novas will join healthcare professionals from around the world who specialize in treatments for conditions and diseases affecting aging and elderly patients from around the world for the anti-aging conference in Mexico City later this month.

Instituto de Medicina Antenvejecimiento Longevidad
The conference will be held at Hotel Reforma in Mexico City.
To learn more or register for the IX Congreso Internacional de Medicina Antienvejecimiento y Longevidad, visit the conference website. For information on Benito Novas and regenerative and stem cell medicine, visit the Global Stem Cells Group website, email info@stemcellsgroup.com, or call +1 305 560 5337.
About Global Stem Cells Group

Global Stem Cells Group (GSCG) is a worldwide network that combines seven major medical corporations, each focused on furthering scientific and technological advancements to lead cutting-edge stem cell development, treatments, and training. The united efforts of GSCG’s affiliate companies provide medical practitioners with a one-stop hub for stem cell solutions that adhere to the highest medical standards.
Global stem cell’s mission is to be the largest recognized stem cell and regenerative medicine network in the world.
Benito Novas keynote speaker
- Published in Press Releases
Global Stem Cells Group names Mehmet Veli Karaaltın, M.D. to Advisory Board
(Image: Mehmet Veli Karaaltın, M.D.)
MIAMI, Jan. 18, 2018—Global Stem Cells Group CEO Benito Novas has named Mehmet Veli Karaaltın, M.D., Associate Professor of Plastic, Aesthetic, and Reconstructive Surgery, (European Board Certified), and hand surgery, head, and maxillofacial surgery specialist to the GSCG Advisory Board Faculty.
Karaaltın practices in Istanbul, Turkey.
Born in Iraq-Kirkuk in 1972, Karaaltın moved to the United States with his family, living in West Lafayette, Indiana and Los Angeles until 1988, when he moved to Istanbul.
After graduating from Istanbul University’s Cerrahpaşa English Medical Faculty, Karaaltın received a national examination from Hacettepe University in Ankara, Turkey, where he was 12th out of 25000 doctoral candidates. In 2012, he passed and completed all ID exams required by the European Society of Plastic Surgery. He is a member of the European Council of Plastic Reconstructive and Aesthetic Surgery and the International Society of Aesthetic Plastic Surgery (ISAPS).
In addition to his work in aesthetic applications, Karaaltın is also known for his work in microvascular free flaps, nerve transfers, facial surgery, and tissue transplants.
Karaaltın also practices cellular therapy and tissue regeneration for healing diabetic wounds, severe burns, and Buerger’s disease. He is one of only a few physicians in the world who offer surgical treatment for Lymphedema-Elephant Disease with microvascular lymph node transfer. In addition, Karaaltın uses
3-D technology to perform aesthetic rhinoplasty.
“It is a pleasure to welcome Dr. Karaaltın to the Global Stem Cells Group Advisory Board,” says GSCG CEO Benito Novas. “He brings a wealth of knowledge in aesthetic regenerative medicine to the table, and we look forward to a long and productive alliance.”
To learn more, visit the Global Stem Cells Group website, email inf@stemcellsgroup.com, or call +1 305 560 5337.
About Global Stem Cells Group:
Global Stem Cells Group (GSCG) is a worldwide network that combines seven major medical corporations, each focused on furthering scientific and technological advancements to lead cutting-edge stem cell development, treatments, and training. The united efforts of GSCG’s affiliate companies provide medical practitioners with a one-stop hub for stem cell solutions that adhere to the highest medical standards.
Global stem cell’s mission is to be the largest recognized stem cell and regenerative medicine network in the world.
Mehmet Veli Karaaltın, M.D.
- Published in Press Releases
ISSCA President Daeyong Kim, Ph.D. Announces Next Chapter Meeting to be Held Jan. 24 – 26, 2018 in Seoul, Korea
ISSCA President Daeyong Kim, Ph.D., and Vice President Benito Novas to hold next ISSCA chapter meeting in Seoul, Korea Jan. 24-26, 2018.
MIAMI, Jan. 18, 2018— ISSCA President Daeyong Kim, Ph.D. has announced the next chapter meeting will be held Jan. 24 – 26, 2018 in Seoul, Korea.
The meeting will be held by Kim and ISSCA Vice President Benito Novas and will address all ISSCA chapter directors and board members.
ISSCA Chapter directors from the U.S., Costa Rica, Argentina, Spain, and Morocco will be in attendance. During the three-day event, chapter directors will receive training in the latest regenerative medicine clinical application protocols as well as applications for manipulating and harvesting stem cells in lab conditions.
ISSCA will also establish strategies for 2018, focusing on promoting excellence and setting standards in the field of publication, research, related education, and certification in regenerative medicine during the event.
The organization intends to set the entire 2018 agenda for regenerative medicine training for 2018 during the meeting, including:
• The Hands on Training Agenda
• The Cell Therapy and Engineering Tissue Fellowship
To learn more about ISSCA, visit the stemcellglobal.org website, email info@stemcellsgroup.com, or call +1305 560 5337.
About ISSCA:

The International Society for Stem Cell Application (ISSCA) is a multidisciplinary community of scientists and physicians, all of whom aspire to treat diseases and lessen human suffering through advances in science, technology and the practice of regenerative medicine. ISSCA serves its members through advancements made to the specialty of regenerative medicine.
The ISSCA’s vision is to take a leadership position in promoting excellence and setting standards in the regenerative medicine fields of publication, research, education, training, and certification.
As a medical specialty, regenerative medicine standards and certifications are essential, which is why ISSCA offers certification training in cities all over the world. The goal is to encourage more physicians to practice regenerative medicine and make it available to benefit patients both nationally and globally. Incorporated under the Republic of Korea as a non-profit entity, the ISSCA is focused on promoting excellence and standards in the field of regenerative medicine.
ISSCA chapter meeting
- Published in Press Releases
ISSCA President Daeyong Kim, Ph.D. Names Junaid Sayed, M.D. President of ISSCA’s U.S. Chapter
(Image: Junaid A. Syed, M.D.)
ISSCA President Daeyong Kim, Ph.D., has named Junaid Sayed, M.D. president of ISSCA’s U.S. chapter.
MIAMI, Jan. 18, 2018—International Society for Stem Cell Application (ISSCA) President Daeyong Kim, Ph.D., has named Junaid Sayed, M.D. president of ISSCA’s U.S. chapter. Sayed is Board Certified physician with extensive training in aesthetic medicine.
As president of the U.S. chapter, Sayed will represent and promote ISSCA activities the USA, including ISSCA’s Stem Cell Certification Training and Stem Cell Conference events. He will also share the latest information on regenerative medicine clinical applications with the U.S, medical community.
“We are happy to have Dr. Sayed represent ISSCA in the U.S., and as a collaborator in our efforts to introduce new clinical applications to the medical community,” says Kim. “He brings a wealth of experience and expertise to the ISSCA organization.”
To learn more about ISSCA, visit the stemcellglobal.org website, email info@stemcellsgroup.com, or call +1305 560 5337.
About ISSCA:

The International Society for Stem Cell Application (ISSCA) is a multidisciplinary community of scientists and physicians, all of whom aspire to treat diseases and lessen human suffering through advances in science, technology and the practice of regenerative medicine. ISSCA serves its members through advancements made to the specialty of regenerative medicine.
The ISSCA’s vision is to take a leadership position in promoting excellence and setting standards in the regenerative medicine fields of publication, research, education, training, and certification.
As a medical specialty, regenerative medicine standards and certifications are essential, which is why ISSCA offers certification training in cities all over the world. The goal is to encourage more physicians to practice regenerative medicine and make it available to benefit patients both nationally and globally. Incorporated under the Republic of Korea as a non-profit entity, the ISSCA is focused on promoting excellence and standards in the field of regenerative medicine.
- Published in Press Releases
Breakthrough in scaling up life-changing stem cell production
Scientists from the U.K. and Sweden have discovered a new method of creating human stem cells that could solve the problem of meeting large-scale production needs, allowing researchers to fully realize the potential of stem cells for understanding and treating disease.
Human pluripotent stem cells are undifferentiated cells that have the unique potential to develop into all the different types of
cells in the body. With applications in disease modeling, drug screening, regenerative medicine and tissue engineering, there is already an enormous demand for these cells, and that demand will continue grow as their use in clinical settings and the pharmaceutical industry increases.
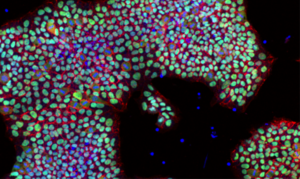
Human embryonic stem cell line HUES1 grown in the new conditions E8+Inter-alpha-inhibitor and imaged for stem cell marker Oct4 (green) and cell-cell attachment molecule E-cadherin (red) with nuclear counter-staining (blue).
Credit: Dr. Sara Pijuan-Galito and Dr. Cathy Merry, Wolfson Centre for Stem Cells, Tissue Engineering & Modelling and Centre for Biomolecular Sciences, The University of Nottingham
Read more at: http://phys.org/news/2016-07-breakthrough-scaling-life-changing-stem-cell.html#jCp
The research results, published in Nature Communications in July, describe how the scientific team from The University of Nottingham’s Wolfson Centre for Stem Cells, Tissue Engineering and Modelling at Uppsala University in Sweden and GE Healthcare also in Sweden have identified and improved human stem cell culture methods that could lead to quicker and cheaper large scale industrial production of human pluripotent stem cells.
By using a protein derived from human blood called Inter-alpha inhibitor, the team has grown human pluripotent stem cells in a minimal medium without the need for costly and time-consuming biological substrates. Inter-alpha inhibitor is found in human blood at high concentrations, and is currently a by-product of standard drug purification schemes.
The human serum-derived protein can make stem cells attach to unmodified tissue culture plastic, eliminating the need for coating in defined human pluripotent stem cell culture, and improving survival capabilities of the stem cells in harsh conditions.
It is the first stem cell culture method that does not require a pre-treated biological substrate for attachment, and therefore, is more cost and time efficient, paving the way for easier and cheaper large-scale production.
Existing methods are time consuming and make developing human stem cell cultures prohibitively costly. This new method has the potential to save time and money in large-scale and high-throughput cultures, and be highly valuable for both basic research and commercial applications.
The work began at Uppsala University, and the study’s first author, Sara Pijuan-Galitó PhD., is continuing her work as a Swedish Research Council Research Fellow at Nottingham.
Researchers now intend to combine Inter-alpha inhibitor protein with an innovative hydrogel technology to improve on current methods for controlling cell differentiation, and also apply it to disease modelling. The discovery, according to the findings, will help facilitate research into many diseases although their focus is currently on understanding rare conditions like Multiple Osteochondroma) at the cellular level. The aim is to replicate the 3 dimensional environment that cells experience within the body so that lab-bench biology is more accurate in modelling diseases.
 Pijuan-Galitó has been awarded the Sir Henry Wellcome Postdoctoral Fellowship at Nottingham University for her work on the research, which will enable her to combine Inter-alpha inhibitor with improved synthetic polymers in collaboration with fellow regenerative medicine pioneers Professor Morgan Alexander and Professor Chris Denning. The team plans to further improve on current human stem cell culture by designing an economical and safe method that can be easily translated to large-scale production and can deliver billions of stem cells necessary move cellular therapeutics forward in patient settings.
Pijuan-Galitó has been awarded the Sir Henry Wellcome Postdoctoral Fellowship at Nottingham University for her work on the research, which will enable her to combine Inter-alpha inhibitor with improved synthetic polymers in collaboration with fellow regenerative medicine pioneers Professor Morgan Alexander and Professor Chris Denning. The team plans to further improve on current human stem cell culture by designing an economical and safe method that can be easily translated to large-scale production and can deliver billions of stem cells necessary move cellular therapeutics forward in patient settings.The study, titled “Human serum-derived protein removes the need for coating in defined human pluripotent stem cell culture,” was published in Nature Communications in July, 2016.
###
- Published in Corporate News / Blog
Global Stem Cells Group to Attend the First Latin American Congress on Sports Medicine in Panama
MIAMI, Sept. 9, 2016—Global Stem Cells Group will attend the Colombian Association of Sports Medicine’s First Latin American Congress on Sports Medicine, Injuries, Treatment and Rehabilitation in Panama City, Panama Sept. 22 – 24, 2016. The Panama Congress will focus on a variety of Sports Medicine specialties presented in an academic activity framework.
The congress will host a diverse range of esteemed guests, including physicians, athletes, athletic coaches, physical therapists, medical exercise physiologists, orthopedists, physical medicine and rehabilitation (PM&R) physicians (physiatrists), Paralympics athletes, endocrinologists, diabetologists, orthopedic surgeons, internists, physical educators, pain specialists, nutrition and dietetic specialists, psychologists, neuro surgeons and neuro rehabilitation specialists.
The event, to be hosted at the Hotel Riu Plaza in the heart of Panama City’s financial district, will provide a dynamic atmosphere showcasing local, national, international and Latin American ambience.
To learn more about the First Latin American Congress on Sports Medicine, visit the event website.To learn more about Global Stem Cells Group, visit the GSCG website, email bnovas(at)stemellsgroup.com, or call 305-560-5337.

About Global Stem Cells Group:
Global Stem Cells Group, Inc. is the parent company of six wholly owned operating companies dedicated entirely to stem cell research, training, products and solutions. Founded in 2012, the company combines dedicated researchers, physician and patient educators and solution providers with the shared goal of meeting the growing worldwide need for leading edge stem cell treatments and solutions. With a singular focus on this exciting new area of medical research, Global Stem Cells Group and its subsidiaries are uniquely positioned to become global leaders in cellular medicine.
To review this press release live online, click here
###
- Published in Press Releases
Global Stem Cells Group Announces Permanent Stem Cell Training Center in South Korea
MIAMI, Aug. 30, 2016 – Global Stem Cells Group and its subsidiary Stem Cell Training, Inc., in a collaborative agreement with South Korean biomedical company N-Biotek, announce the establishment of a permanent stem cell training center at the N-Biotek headquarters in Seoul, South Korea. The announcement comes as part of an ambitious expansion effort between GSCG and N-Biotek to provide stem cell training to qualified physicians worldwide.
Stem Cell Training’s two-day intensive “Adipose-derived Stem Cell and Bone Marrow and Platelet Rich Plasma (PRP)” training course covers concepts in cellular medicine, cell viability basics, basic fat and bone marrow stem cell harvesting, isolation and processing procedures, and principles of platelet rich plasma (PRP) processing.
The Korean training center will offer GSCG’s full array of Stem Cell Training courses including:
The “Cell Assisted Fat Transfer” training course is designed for physicians in the aesthetic and cosmetic fields of medicine. The course specifically focuses on procedures to address facial aging caused by volume loss. Course training covers the basics of facial anatomy, facial aging, and the use of fat harvested from the patient’s own body as a filler.
The “Cell Assisted Fat Transfer” training prepares the physician to perform a mini liposuction procedure in order to retrieve fat for grafting in the patient’s anti-aging treatment. It also provides instruction on placement techniques for female and male patients according to age and ethnicity. Fat is a popular filler in the aesthetic medicine field, as the industry experiences an increase in liposuction procedures, an increased interest in facial re-volumization and body, and a growing appreciation for the regenerative potential of adipose-derived mesenchymal stem cells.
The “Diplomat Cell Therapy and Tissue Engineering” course is offered to physicians and qualified practitioners interested in implementing regenerative medicine therapies. This course offers a detailed, hands-on experience in stem cell characterization and laboratory applications; cell protocols including culture, plating, trypsinization, harvesting and cryopreservation; instruction in applying quality control tests including cell count, viability, flow cytometry, endotoxin, mycoplasma and sterility; the ability to perform cGMP functions including clean room maintenance, gowning and environmental monitoring, and new insight and relevant applications of stem cell processing and regulations in a certified facility.
The “Online SVF and Bone Marrow” training course is an online stem cell training course that provides physicians an opportunity to take the clinical training course in a web based format presented as video and voiced over slides. The online course content is consistent with Stem Cell Training’s clinical training course, as it focuses on autologous cellular treatments such as adult stem cells and platelet rich plasma (PRP). Attendees learn how to extract lipoaspirate and bone marrow aspirate, and how to isolate adipose and bone marrow derived stem cells.
In addition, all techniques and materials presented in the training videos are accompanied with detailed protocols.
This course also provides attendees with the tools necessary to implement regulatory and clinical guidelines when setting up a GMP facility; copies of presentations, procedural protocols and all forms associated with a GMP facility; the ability to perform clinical procedures including lipoaspirate and bone marrow isolation; the ability to reintroduce stem cells for various indications, and case books and full protocols for approximately 30 indications.
The two-day online clinical course includes in-clinic procedural videos, recorded didactic lectures, additional information and videos on regenerative medicine, and digital versions of course procedures, protocols and videos.
Stem Cell Training’s experienced and knowledgeable trainers will cover indications, contraindications, patient selection, pre- and post-treatment instructions, and treatment alternatives for autologous facial fat transfer. In addition, participants will learn to analyze anatomy for appropriate areas of facial enhancement; learn various injection techniques to correct temporal hollows, volume loss, tear trough deformity, pre jowl sulcus, malar fat pad, submalar areas, and melomental and nasolabial folds; learn to revent and manage cell-assisted autologous facial fat transfer complications, and understand anesthetic techniques.
To learn more about the stem cells training course center in South Korea, visit the Global Stem Cells Group website, or the Stem Cell Training website, email bnovas@stemcellsgroup.com, or call +1 305 560 5337
About Global Stem Cell Group:
Global Stem Cells Group is the parent company of six wholly owned operating companies dedicated entirely to stem cell research, training, products and solutions. Founded in 2012, the company combines dedicated researchers, physician and patient educators and solution providers with the shared goal of meeting the growing worldwide need for leading edge stem cell treatments and solutions. With a singular focus on this exciting new area of medical research, Global Stem Cells Group and its subsidiaries are uniquely positioned to become global leaders in cellular medicine.
 About Stem Cell Training, Inc.:
About Stem Cell Training, Inc.:
Stem Cell Training, Inc. is a multi-disciplinary company offering coursework and training in 35 cities worldwide. The coursework offered focuses on minimally invasive techniques for harvesting stem cells from adipose tissue, bone marrow and platelet-rich plasma. By equipping physicians with these techniques, the goal is to enable them to return to their practices, better able to apply these techniques in patient treatments.
About N-Biotek:
Since 1982, N-Biotek has been the leading manufacturer of biomedical and lab equipment worldwide. The Stem Cell Total Solution lab is a culmination of years  of dedication to establish compact and customized products in developing the Handy Lab-a personal lab for medical, scientific and university professionals. N-Biotek is known for its high quality, uniquely designed biomedical products and equipment and competitive pricing. Over the years, the company’s products have earned an array of patents, and all meet international standards including CE, ETL, ISO and GMP. N-Biotek also offers real-time monitoring through IT technology.
of dedication to establish compact and customized products in developing the Handy Lab-a personal lab for medical, scientific and university professionals. N-Biotek is known for its high quality, uniquely designed biomedical products and equipment and competitive pricing. Over the years, the company’s products have earned an array of patents, and all meet international standards including CE, ETL, ISO and GMP. N-Biotek also offers real-time monitoring through IT technology.
Since 2010, N-Biotek has expanded significantly, starting new businesses including a stem cell processing system and biological clean room, GMP consulting, validation services and health care services for foreign consumers in order to maintain its lead in the life science field.
To view this press release live online, click here
###
- Published in Press Releases

![ISSCA_RAFIK[1]](https://stemcellcenter.net/wp-content/uploads/2018/03/ISSCA_RAFIK1-460x260_c.png)
![ISTANBUL_TURKEY[1]](https://stemcellcenter.net/wp-content/uploads/2018/03/ISTANBUL_TURKEY1-460x260_c.png)
![GSCG_TRAINING_MEXICO4[1]](https://stemcellcenter.net/wp-content/uploads/2018/03/GSCG_TRAINING_MEXICO41-460x260_c.png)
![BENITO2[1]](https://stemcellcenter.net/wp-content/uploads/2018/03/BENITO21-460x260_c.png)

![STEM_CELL_TRAINING[1]](https://stemcellcenter.net/wp-content/uploads/2018/03/STEM_CELL_TRAINING1-460x260_c.png)
![Junaid-A.-Syed-MD2[1]](https://stemcellcenter.net/wp-content/uploads/2018/03/Junaid-A.-Syed-MD21-460x260_c.png)