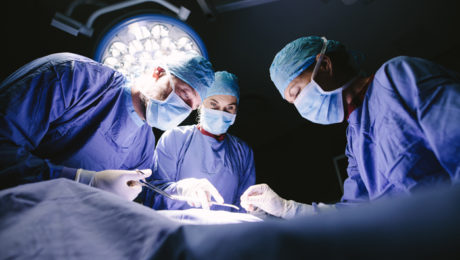
ISSCA to Conduct Sponsored Regenerative Medicine Training Course in Caracas, Venezuela Dec. 2-3, 2018
ISSCA will conduct a hands-on regenerative medicine certification training course for physicians in Caracas, Venezuela Dec. 2-3, 2018. Global Stem Cells Group will sponsor 50 percent of the training fees to provide most physicians in economically-strapped Venezuela with access to regenerative medicine education.
MIAMI, Nov. 1, 2018—The International Society for Stem Cell Application (ISSCA) announces plans to hold a regenerative medicine certification training course in Caracas, Venezuela Dec. 2-3, 2018. The two-day course teaches easy-to-follow protocols for harvesting adipose and bone marrow stem cells from patients in a clinical environment..
The training program follows the organization’s sponsored regenerative medicine symposium in Caracas on Dec. 1, 2018. ISSCA affiliate Global Stem Cells Group Group is sponsoring the training program and funding 50 percent of fee costs to ensure most Venezuelan physicians can attend.
GSCG CEO and ISSCA Vice President Benito Novas says the training program is an important part of the organizations’ commitment to spreading knowledge and physician training in regenerative medicine to medical professionals worldwide. This commitment includes strategies to accommodate countries like Venezuela that lack the resources to train physicians to implement regenerative medicine procedures in clinical settings.
“Our goal is to make economically and therapeutically viable regenerative medicine therapies available to Venezuelans by training and educating physicians throughout the country in stem cell therapies and protocols they can provide to patients in their clinics, says Novas. “We’re driven by our conviction that stem cell medicine will bring transformative changes to the healthcare industry at a global level.
Participating physicians learn how to harvest and isolate bone marrow and adipose-derived autologous stem cells to use in treating multiple conditions.
The two-day course begins with a four hour lecture on advances in regenerative medicine. Participating physicians will receive more than 12 hours of hands-on training with live patients in protocols for obtaining stem cells from patients that can be used in the medical office. They will also receive 12 protocols in digital format to take back to their practices and begin using stem cell applications immediately.
The course provides participating physicians with training in stem cell therapies to treat a variety of conditions. Participating physicians gain Knowledge and practical skills to easily add regenerative medicine to their practices.
Physicians will also receive access to the online stem cell training course to review all content and procedures introduced in the 2-day clinical training course, patient forms and guidelines, procedure informed consent forms, didactic lectures, training booklets and more.
ISSCA’s stem cell certification course offers practical, personalized training in stem cell harvesting and isolation..ISSCA has trained more than 2,000 physicians worldwide.
Stem cell therapies continue to revolutionize the healthcare industry and help improve the quality of life for patients.
To learn more about the ISSCA certification training course and to register to participate, visit the Stem Cells Training website, the email info@stemcellsgroup.com, or call 305-560-5337.
About ISSCA:

The International Society for Stem Cell Application (ISSCA) is a multidisciplinary community of scientists and physicians, all of whom aspire to treat diseases and lessen human suffering through advances in science, technology and the practice of regenerative medicine. ISSCA serves its members through advancements made to the specialty of regenerative medicine.
The ISSCA’s vision is to take a leadership position in promoting excellence and setting standards in the regenerative medicine fields of publication, research, education, training, and certification.
As a medical specialty, regenerative medicine standards and certifications are essential, which is why ISSCA offers certification training in cities all over the world. The goal is to encourage more physicians to practice regenerative medicine and make it available to benefit patients both nationally and globally. Incorporated under the Republic of Korea as a non-profit entity, the ISSCA is focused on promoting excellence and standards in the field of regenerative medicine.
About Global Stem Cells Group: Global Stem Cells Group (GSCG) is a worldwide network that combines seven major medical corporations. Each corporation is focused on furthering scientific and technological advancements in cutting-edge stem cell research, development, treatment, and training. The united efforts of GSCG’s affiliate companies provide medical practitioners with a one-stop epicenter for stem cell solutions that adhere to the highest medical standards.
Global Stem Cells Group (GSCG) is a worldwide network that combines seven major medical corporations. Each corporation is focused on furthering scientific and technological advancements in cutting-edge stem cell research, development, treatment, and training. The united efforts of GSCG’s affiliate companies provide medical practitioners with a one-stop epicenter for stem cell solutions that adhere to the highest medical standards.
Global stem cell’s mission is to be the largest recognized stem cell and regenerative medicine network in the world. About Adimarket: Adimarket, Inc., a division of the Global Stem Cells Group, is a one-stop, cost-competitive online marketplace for quality regenerative medicine equipment and supplies for physicians and healthcare professionals.
###
Caracas Venezuela stem cell training
- Published in Press Releases
Global Stem Cells Group Announces New Business Development Director for Africa and Europe

Holya Aziza El Materi Ep Khemiri, M.D.
Global Stem Cells Group announces the appointment of Holya Aziza El Materi Ep Khemiri, M.D. as Vice President Business Development Africa and Europe. Khemiri will oversee development of a new Stem Cell Center in Tunisia.
MIAMI, Nov. 1, 2018—Global Stem Cells Group founder and CEO Benito Novas has named Holya Aziza El Materi Ep Khemiri, M.D. Vice President Business Development for Africa and Europe. The announcement is the latest update on the regenerative medicine organization’s expansion of its clinical presence on the two continents.
Khemiri, who specializes in pharmaceutical and parapharmacy medicines, product manufacturing and medical industry business development, will be in charge of developing and opening a new Stem Cell Center in Tunisia focused on the treatment of patients with degenerative illnesses and orthopedic impairments, and professional athletes with sports injuries.
Stem Cell Center Tunisia will provide regenerative medicine training to African physicians who wish to offer stem cell therapies in their practices.
Since opening the first African Stem Cell Center in Casablanca, Morocco in July, 2017, GSCG has accelerated momentum to bring regenerative medicine, training and education to Africa and Europe through expanded networking opportunities introducing the organization’s authoritative training programs, workshops, seminars, online marketplace for regenerative medicine products, expert instruction and advice on marketing for regenerative practices and other innovative initiatives.
Stem Cell Center Tunisia is part of Global Stem Cells Group’s strategy to facilitate international growth and bring quality-of-life-enhancing regenerative medicine to patients in every corner of the world. The Tunisian center’s medical team will work closely with regional patients, educators and regulators to foster engagement and promote stem cell medicine technology.
ction as a regenerative medicine reference for physicians in North Africa, providing physicians with instruction in stem cell therapies and protocols Those wishing to implement stem cell oversight in their practices will be able to visit the office and collaborate other physicians, participate in educational classes and workshops, and receive the training necessary to start implementing regenerative medicine in their practices and clinics.
Additionally, Stem Cell Center Tunisia will offer physicians a stem cell processing center, GSCG’s complete solution for onsite tissue processing, isolation, culturing and cryopreservation of stem cells. One of the biggest challenges of using traditional stem cell harvesting protocols in medical office settings is obtaining enough stem cells to treat certain degenerative conditions such as autism, lupus. Parkinson’s Disease, Alzheimer’s Disease and others.
The stem cell processing center provides a solution that allows physicians to expand stem cell colonies and obtain a significantly larger number of cells sufficient to treat patients. It also helps with patients needing multiple stem cell treatments by allowing the physician to perform a single extraction of stem cells from fat tissue; the cells harvested can then be saved and expanded, eliminating the need for new cell extraction with each treatment.
According to Novas, Khemiri’s skills and experience make her an ideal candidate to spearhead Stem Cell Center Tunisia’s development and opening.
“Dr. Khemiri brings an array of expertise and knowledge to our expansion efforts in Africa and Europe,” says Novas. She joins Global Stem Cells Group’s management team as a welcome addition at a time when our international expansion endeavors are growing exponentially.”
Khemiri was president of Tunisia’s Society Isotope Radioavtive (SISORA) from 2011 to 2014, where she was involved in manufacturing radioactive tracers to detect cancer. As president of the organization, she helped establish a partnership between SISORA and Belgium-based Radio Pharma Solultions (IBA).
From 2008 to 2011, she served as Research and Development Director of ADWYA, a Tunisia-based company specializing in the production of pharmaceutical products for medical and veterinary use and the country’s first private pharmaceutical laboratory and one of its largest private pharmaceutical companies.
In 2010, she served as creative director of RAD where she focused on formula and scientific studies and registration of medicines, skin care and food supplements with the Tunisian Health Ministry.
Khemari received her medical training at the Catholic University of Louvain in Brussels, Belgium from 1999 to 2006. She received a diploma in Pharmaceutical Sciences from the the Institut Roger Lambion in Brussels and a diploma in Biochemistry, Cosmetology, and Science Perfumery from the University Libre in Brussels.
For more information, visit the Global Stem Cells Group website, email info@stemcellsgroup.com or call 305-560-5337.
About Global Stem Cells Group:
Global Stem Cells Group (GSCG) is a worldwide network that combines seven major medical corporations. Each corporation is focused on furthering scientific and technological advancements in cutting-edge stem cell research, development, treatment, and training. The united efforts of GSCG’s affiliate companies provide medical practitioners with a one-stop epicenter for stem cell solutions that adhere to the highest medical standards.
Global stem cell’s mission is to be the largest recognized stem cell and regenerative medicine network in the world. About Adimarket: Adimarket, Inc., a division of the Global Stem Cells Group, is a one-stop, cost-competitive online marketplace for quality regenerative medicine equipment and supplies for physicians and healthcare professionals.
###
Holya Aziza El Materi Ep Khemiri named Vice President Business Development Africa and Europe.
- Published in Press Releases
Global Stem Cells Group launches “Regenerative Medicine” video blog on YouTube
Global Stem Cells Group announces the launch of the new “Regenerative Medicine” vlog featuring interviews with leaders in regenerative medicine.
MIAMI, Sept. 14, 2018–Global Stem Cells Group (GSCG), a word leader in regenerative medicine, has launched a new video blog (vlog) titled “Regenerative Medicine” on its YouTube channel. Physicians who incorporate regenerative medicine therapies in their patient treatments are invited to participate in the vlog.
The idea is to create a space for regenerative medicine practitioners to share their clinical experiences with viewers around the world. New interviews will be recorded and aired weekly on the “Regenerative Medicine” vlog.
The new vlog channel is part of Global Stem Cells Group’s ongoing effort to spread the word about the benefits of regenerative medicine therapies to viewers around the world and to provide practitioners with an opportunity to discuss their regenerative medicine practices with interested audiences, and stay abreast of what other practitioners in the field are doing.
According to Benito Novas, GSCG CEO, the vlog platform will provide an additional point of reference for physicians and patients looking for the latest information on stem cell research and therapies. It is also designed to help clarify the scope of stem cell procedures and to dispel myths and false information among audiences. All physicians who wish to participate in GSCG’s new vlog program will be included and encouraged to speak freely.
Physicians wishing to participate in an interview for the GSCG “Regenerative Medicine” vlog series are asked to email their CV and abstract to info@stemcellsgroup,com.
The Regenerative Medicine vlog will be posted on Global Stem Cells Group’s YouTube Channel and physician interviews will also be posted on the News ABout Cell Therapies blog.
For more information about Global Stem Cells Group, visit the GSCG website, email: info@stemcellsgroup.com, or call +1305 560 5337.
About Global Stem Cells Group:
Global Stem Cells Group (GSCG) is a worldwide network that combines seven major medical corporations. Each corporation is focused on furthering scientific and technological advancements in cutting-edge stem cell research, development, treatment, and training. The united efforts of GSCG’s affiliate companies provide medical practitioners with a one-stop epicenter for stem cell solutions that adhere to the highest medical standards.
Global stem cell’s mission is to be the largest recognized stem cell and regenerative medicine network in the world
- Published in Press Releases
Regenerative medicine symposium hosted by ISSCA in Buenos Aires, Argentina Aug. 24, 2018, exceeds expectations organizers report
Organizers of the ISSCA regenerative medicine symposium “Applications of Cell Therapies in Medicine and Aesthetic Surgery” held in Buenos Aires, Argentina Aug. 24, 2018, featured 16 physicians traveling from six countries— Mexico, Chile, Colombia, Spain, Costa RIca and the U.S. —to serve as guest speakers.
MIAMI, Aug. 30, 2018—Enthused by the extraordinary success of the International Association for Stem Cell Applications (ISSCA)’s regenerative medicine symposium held at Universidad de Buenos Aires in Argentina Aug. 24, 2018, ISSCA organizers have already begun discussing plans to host the next conference in Miami in summer, 2019.
The 5th annual “Applications of Cell Therapies in Medicine and Aesthetic Surgery,” hosted 250 attendees and featured more than 16 speakers from six countries to discuss the latest clinical stem cell applications for a variety of therapies and treatments including traumatic injuries, pain management, neurological conditions, sports injuries, heart failure, erectile effusion, and aesthetic medicine procedures.
The symposium provided attendees with an opportunity to learn about the most important clinical applications of stem cell therapies from experienced regenerative medicine practitioners.
Leonardo Tacus, M.D., an arthroscopic surgeon specializing in orthopedics, traumatology, and regenerative medicine, spoke to attendees about clinical applications of regenerative medicine therapies to treat injuries sustained by professional athletes.
Jose Benitez, Medical Director of Boston Médical Group Spain. who specializes in male sexual dysfunctions. spoke about stem cell applications used to treat erectile dysfunction.
Calling the symposium their most successful regenerative medicine event to date, iSSCA members and conference speakers held an informative Q&A session lasting three hours until all questions were answered. The session covered a variety of compelling topics regarding stem cell therapies. A lecture based on the symposium will soon be available on ISSCA’s YouTube channel.
Information on the summer, 2019 symposium will be made available in upcoming weeks. For more information, visit stemcellconference.org website, email info@stemcellsgroup.com, or call +1305 560 5337.
About ISSCA: International Society for Stem Cell Application (ISSCA) is a multidisciplinary community of scientists and physicians, all of whom aspire to treat diseases and lessen human suffering through advances in science, technology and the practice of regenerative medicine. ISSCA serves its members through advancements made to the specialty of regenerative medicine.
International Society for Stem Cell Application (ISSCA) is a multidisciplinary community of scientists and physicians, all of whom aspire to treat diseases and lessen human suffering through advances in science, technology and the practice of regenerative medicine. ISSCA serves its members through advancements made to the specialty of regenerative medicine.
The ISSCA’s vision is to take a leadership position in promoting excellence and setting standards in the regenerative medicine fields of publication, research, education, training. and certification.
As a medical specialty, regenerative medicine standards and certifications are essential, which is why ISSCA offers certification training in cities all over the world. The goal is to encourage more physicians to practice regenerative medicine and make it available to benefit patients both nationally and globally. Incorporated under the Republic of Korea as a non-profit entity, the ISSCA is focused on promoting excellence and standards in the field of regenerative medicine.
###
regenerative medicine symposium Buenos Aires
- Published in Press Releases
ISSCA to sponsor International Congress on Advanced Therapies in Medicine in Riviera Nayarit, Mexico Nov. 24-25, 2018
ISSCA will sponsor the 6th annual International Congress on Advanced Therapies in Medicine Nov. 24-25, 2018 in Riviera Nayarit.
MIAMI, July 27, 2018—For the third consecutive year the International Association for Stem Cell Application (ISSCA) will sponsor the International Congress on Advanced Therapies in Medicine 2018 on Puerto Vallarta, Mexico.
The event will feature a commercial expo with 20 with 20 booths exhibiting new products and information for physicians in the field of regenerative medicine. More than 250 physicians are expected to be in attendance.
ISSCA will present information on its Fellowship in Cell Therapy and Tissue Engineering program, a 6-day course during which a group of experienced academicians involved in stem cell transplantation present the general principles and practice of stem cell biology and evidence-based treatments to physicians looking to optimize the treatment options that benefit the health of their patients. The fellowship program will be held in Korea Oct. 22-27, 2018.
Fellowship participants will take part in a detailed program offering hands-on experience in stem cell applications and learn cell culture protocols including plating, trypsinization, harvesting, and cryopreservation, as well as an understanding and application of quality control tests including cell count, viability, flow cytometry, endotoxin, mycoplasma and sterility.
In addition, physicians participating in the ISSCA fellowship program will learn how to perform cGMP functions including cleanroom maintenance, gowning and environmental monitoring, while gaining insight on relevant applications of stem cell processing and regulations in a certified facility. ISSCA provides fellowship attendees with the tools necessary to implement regulatory and clinical guidelines when setting up a GMP facility, as well as copies of presentations, procedural protocols and all forms associated with a GMP facility provided.
Participating physicians learn to perform clinical procedures including lipoaspirate and bone marrow isolation, and reintroducing stem cells for various indications. Case books and full protocols for approximately 30 indications are also provided.
In addition, Global Stem Cells Group )GSCG) will present its stem cell processing center, a complete solution for providers to equip their practice facilities with an on-site regenerative medicine lab. Used for tissue processing, isolation, culturing and cryopreservation of stem cells, the stem cell processing center is designed to execute a state-of-the-art stem cell laboratory using the latest technology.
GSCG provides participating regenerative medicine teams with specialized training to implement high-level processes and therapies using the stem cell processing center for better clinical interpretation, better results while maintaining compliance with safety regulations and international standards.
GSCG’s stem cell laboratory solution represents a tremendous competitive advantage and differentiator that arms regenerative medicine practitioners with the ability to perform highly advanced stem cell procedures in their facilities.
The 2018 International Congress on Advanced Therapies in Medicine in Riviera Nayarit, Mexico will be held at the Hotel Krystal Grand in Nuevo Puerto Vallarta,
For more information and to register, email info@stemcellsgroup.com, or call +1305 560 5337.
About ISSCA:
 The International Society for Stem Cell Application (ISSCA) is a multidisciplinary community of scientists and physicians, all of whom aspire to treat diseases and lessen human suffering through advances in science, technology and the practice of regenerative medicine.
The International Society for Stem Cell Application (ISSCA) is a multidisciplinary community of scientists and physicians, all of whom aspire to treat diseases and lessen human suffering through advances in science, technology and the practice of regenerative medicine.
ISSCA serves its members through advancements made in the specialty of regenerative medicine.ISSCA’s vision is to take a leadership position in promoting excellence and setting standards in the regenerative medicine fields of publication, research, education, training, and certification.
As a medical specialty, regenerative medicine standards and certifications are essential, which is why ISSCA offers certification training in cities all over the world. The goal is to encourage more physicians to practice regenerative medicine and make it available to benefit patients both nationally and globally. Incorporated under the Republic of Korea as a non-profit entity, the ISSCA is focused on promoting excellence and standards in the field of regenerative medicine.
About Global Stem Cells Group:
Global Stem Cells Group (GSCG) is a worldwide network that combines seven major medical corporations. Each corporation is focused on furthering scientific and technological advancements in cutting-edge stem cell research, development, treatment, and training. The united efforts of GSCG’s affiliate companies provide medical practitioners with a one-stopepicenter for stem cell solutions that adhere to the highest medical standards.
major medical corporations. Each corporation is focused on furthering scientific and technological advancements in cutting-edge stem cell research, development, treatment, and training. The united efforts of GSCG’s affiliate companies provide medical practitioners with a one-stopepicenter for stem cell solutions that adhere to the highest medical standards.
Global stem cell’s mission is to be the largest recognized stem cell and regenerative medicine network in the world.
advanced therapies international congress
- Published in Press Releases
ISSCA announces fellowship in cell therapy and tissue engineering
As part of its ongoing efforts to meet growing demand among physicians for continuing education opportunities in advanced cell therapies and tissue banking, the International Society for Stem Cell Application (ISSCA) will hold its next Cell Therapy and Tissue Engineering Fellowship program in Seoul, Korea October 22 – 27, 2018.
ISSCA’s fellowship training program offers physicians an opportunity to build knowledge and increase their therapeutic skills by learning to employ new developments in tissue banking practices and advanced cellular therapies to utilize in their practices.
The fellowship training will enable participating physicians to improve their core skills and competencies in regenerative medicine, establish optimal protocols, policies, and practices in tissue, cell, and advanced therapies, and ultimately enable them to deliver superior services to their patients.
Participating physicians will have the advantage of active engagement with world-class cellular and tissue banking experts in an immersive, hands-on experience while learning theoretical and analytic methods. Fellowship mentors are seasoned professionals devoted to creating new medical technologies and laboratory applications in one of the most rapidly-growing areas of biomedical engineering.
Cell therapy and tissue engineering offer tremendous potential for a career as a practitioner or researcher. Today’s students can be tomorrow’s pioneers in improving the efficacy of medical treatments and advancing the healthcare industry.
Engineering human tissues and organs can provide new treatments and cures for diseases, including blood vessels, bone, cartilage, liver, pancreas, peripheral nerves, and skin cells. Cell therapies and tissue engineering have the potential to revolutionize disease remediation and tissue repair in miraculous ways.
Fellowship participants will have complete access to ISSCA’s accredited stem cell advanced lab protocols. The ISSCA Fellowship program is a 6-day program that offers a certificate of completion issued by ISSCA and Westminster International University in Seoul. Students who complete the fellowship become members of the ISSCA’s international network of regenerative medical professionals and standard setters, with access to events, resources, training, and support moving forward.
ISSCA is the only organization that provides a complete cell therapy and tissue engineering fellowship focused on regenerative medicine. It is also the only fellowship of its kind supported by Korean Universities.
To learn more about the ISSCA fellowship and to reserve a spot at the October 2018 program in Korea, visit the Fellowship in Cell Therapy and Tissue Engineering website, email info@stemcellsgroup.com, or call +1 813 510 9403
About ISSCA:
 The International Society for Stem Cell Application (ISSCA) is a multidisciplinary community of scientists and physicians, all of whom aspire to treat diseases and lessen human suffering through advances in science, technology and the practice of regenerative medicine. ISSCA serves its members through advancements made in the specialty of regenerative medicine.
The International Society for Stem Cell Application (ISSCA) is a multidisciplinary community of scientists and physicians, all of whom aspire to treat diseases and lessen human suffering through advances in science, technology and the practice of regenerative medicine. ISSCA serves its members through advancements made in the specialty of regenerative medicine.
The ISSCA’s vision is to take a leadership position in promoting excellence and setting standards in the regenerative medicine fields of publication, research, education, training, and certification.
As a medical specialty, regenerative medicine standards and certifications are essential, which is why ISSCA offers certification training in cities all over the world. The goal is to encourage more physicians to practice regenerative medicine and make it available to benefit patients both nationally and globally. Incorporated under the Republic of Korea as a non-profit entity, the ISSCA is focused on promoting excellence and standards in the field of regenerative medicine.
###
cell therapy fellowship
- Published in Press Releases
ISSCA introduces new stem cell training courses web page for regenerative medicine practitioners
ISSCA has launched a new stem cell training web page designed to offer free information and resources to help physicians choose a training program best suited to their unique needs.
MIAMI, June 26, 2018—The International Society for Stem Cell Application (ISSCA) has launched a new stem cell training course web page, coordinated by Global Stem Cells Group affiliate Stem Cell Training, designed to help physicians access free information and resources on the newest instruction and training options in regenerative medicine training.
The new web page is designed to help physicians interested in adding stem cell procedures to grow their medical practice or enhance career advancement opportunities find stem cell training program options to enable them to find a training program best suited to their individual needs. ISSCA’s variety of stem cell training opportunities include:
• Online stem cell training course, ISSCA’s cutting-edge online course that teaches physicians everything they need to know to add adult stem cell-based procedures to their existing practice, or confidently transition to a regenerative medicine center. Offering the convenience of training from a home or office computer, this course prepares physicians in all the theoretical and practical knowledge needed to effectively and expertly administer stem cell therapies to patients, including harvesting and isolating stem cells.
ISSCA’s online training positions physicians to open their own stem cell center practice and join ISSCA’s expansive network. Successful completion of the online training course allows physicians to immediately begin offering cutting-edge regenerative medicine procedures to patients, establish themselves as experts in their fields, and enjoy the benefits of the growing regenerative medicine industry.
• Hands-on stem cell certification training courses, ISCCA’s intensive, two-day hands-on training course scheduled at various international locations provides attending physicians with expert instruction on autologous stem cell therapies in the field of regenerative medicine. Participants learn techniques and protocols for harvesting and isolating stem and regenerative cells from adipose tissue, bone marrow, and /or peripheral blood from live patients and administering the cells back to the patient
Course curriculum consists of comprehensive theoretical lectures and home study education, and two days of didactic and clinical experience. One day of post-educational on-site clinical assistance is also available upon request.
• Onsite training, ISSCA’s personalized, hands-on, onsite stem cell training brings stem cell specialists to your practice or clinic, anywhere in the world, to provide one-on-one training tailored to your practice’s specific requirements—saving time and money. The onsite training program offers participants a unique opportunity to grow their practice and achieve their specific practice goals by offering practice-specific regenerative medicine treatments to patients in their medical office or clinical setting.
The onsite training course provides participating practices with personalized theoretical information and hands-on training along with ongoing support for their clinical practice. Applications and protocols are provided by a Stem Cells Training faculty member with extensive experience in laboratory and clinical practice.
ISSCA’s onsite training specifications include:
1. Equipment and supply delivery. The Stem Cell Training team
delivers and sets up all equipment and supplies necessary for the training session to take place and will leave the physician’s team
fully qualified to start its own stem cell treatment practice.
2. Expert trainers. ISSCA’s onsite stem cell training course takes a highly visual and interactive approach. Expert trainers teach and supervise the hands-on process using live patients and different protocols for the extraction, isolation, and application of PRP, adipose- and bone marrow-derived stem cells.
3. Multimedia access. ISSCA provides physicians participating in its onsite training program access to its library of high-resolution, step-by-step procedure videos and ongoing online and telephone support for clinical equipment, inquiries or concerns for the practice’s future use and reference.
• Fellowship in cell therapy and tissue engineering. Recognizing the need for knowledge of stem cell protocols among physicians and healthcare professionals, ISSCA and Stem Cell Training created the Fellowship of Stem Cell Therapy and Tissue Engineering program. The fellowship focuses on stem cell therapies involving the potential replacement of cells or organs that are diseased, injured, infirmed, ailing or aged
In this modular training program, a group of experienced academic scholars involved in stem cell transplantation present a series of topics covering the general principles and practices of stem cell
biology and evidence-based treatments that physicians can apply to optimize the health of their patients. Fellowship course details and objectives include:
• A detailed program offering hands-on experience in stem cell characterization and laboratory applications
• An opportunity to learn cell culture processes including plating, trypsinization, harvesting, and cryopreservation
• Gaining the ability to understand and apply quality control tests including cell count, viability, flow cytometry, endotoxin, mycoplasma, and sterility
• Learning to perform CGMP functions including clean room maintenance, gowning, and environmental monitoring
• Establishing insight on relevant applications of stem cell processing and regulations that apply to a certified facility
• Receiving the tools necessary to implement regulatory and clinical guidelines when setting up a GMP facility
• Providing participants with copies of presentations, procedural protocols, and all forms associated with a GMP facility, as well as case books and full protocols for approximately 30 indications
• Demonstrating the ability to perform clinical procedures including lipoaspirate and bone marrow isolation, and reintroduction of stem cells for various indications
The new ISSCA stem cell training web page also features an informative blog that publishes four new articles in the field of regenerative medicine weekly.
To learn more, visit the ISSCA stem cell training web page, email info@stemcellsgroup.com, or call 305-560-5337.
About ISSCA:
The International Society for Stem Cell Application (ISSCA) is a multidisciplinary community of scientists and physicians, all of whom aspire to treat diseases and lessen human suffering through advances in science, technology and the practice of regenerative medicine. ISSCA serves its members through advancements made in the specialty of regenerative medicine.
The ISSCA’s vision is to take a leadership position in promoting excellence and setting standards in the regenerative medicine fields of publication, research, education, training, and certification.
As a medical specialty, regenerative medicine standards and certifications are essential, which is why ISSCA offers certification training in cities all over the world. The goal is to encourage more physicians to practice regenerative medicine and make it available to benefit patients both nationally and globally. Incorporated under the Republic of Korea as a non-profit entity, ISSCA is focused on promoting excellence and standards in the field of regenerative medicine.
Stem cell training web page launch
- Published in Press Releases
ISSCA introduces new stem cell training courses web page for regenerative medicine practitioners
ISSCA has launched a new stem cell training web page designed to offer free information and resources to help physicians choose a training program best suited to their unique needs.
MIAMI, June 26, 2018—The International Society for Stem Cell Application (ISSCA) has launched a new stem cell training course web page, coordinated by Global Stem Cells Group affiliate Stem Cell Training, designed to help physicians access free information and resources on the newest instruction and training options in regenerative medicine training.
The new web page is designed to help physicians interested in adding stem cell procedures to grow their medical practice or enhance career advancement opportunities find stem cell training program options to enable them to find a training program best suited to their individual needs. ISSCA’s variety of stem cell training opportunities include:
• Online stem cell training course, ISSCA’s cutting-edge online course that teaches physicians everything they need to know to add adult stem cell-based procedures to their existing practice, or confidently transition to a regenerative medicine center. Offering the convenience of training from a home or office computer, this course prepares physicians in all the theoretical and practical knowledge needed to effectively and expertly administer stem cell therapies to patients, including harvesting and isolating stem cells.
ISSCA’s online training positions physicians to open their own stem cell center practice and join ISSCA’s expansive network. Successful completion of the online training course allows physicians to immediately begin offering cutting-edge regenerative medicine procedures to patients, establish themselves as experts in their fields, and enjoy the benefits of the growing regenerative medicine industry.
• Hands-on stem cell certification training courses, ISCCA’s intensive, two-day hands-on training course scheduled at various international locations provides attending physicians with expert instruction on autologous stem cell therapies in the field of regenerative medicine. Participants learn techniques and protocols for harvesting and isolating stem and regenerative cells from adipose tissue, bone marrow, and /or peripheral blood from live patients and administering the cells back to the patient
Course curriculum consists of comprehensive theoretical lectures and home study education, and two days of didactic and clinical experience. One day of post-educational on-site clinical assistance is also available upon request.
• Onsite training, ISSCA’s personalized, hands-on, onsite stem cell training brings stem cell specialists to your practice or clinic, anywhere in the world, to provide one-on-one training tailored to your practice’s specific requirements—saving time and money. The onsite training program offers participants a unique opportunity to grow their practice and achieve their specific practice goals by offering practice-specific regenerative medicine treatments to patients in their medical office or clinical setting.
The onsite training course provides participating practices with personalized theoretical information and hands-on training along with ongoing support for their clinical practice. Applications and protocols are provided by a Stem Cells Training faculty member with extensive experience in laboratory and clinical practice.
ISSCA’s onsite training specifications include:
1. Equipment and supply delivery. The Stem Cell Training team
delivers and sets up all equipment and supplies necessary for the training session to take place and will leave the physician’s team
fully qualified to start its own stem cell treatment practice.
2. Expert trainers. ISSCA’s onsite stem cell training course takes a highly visual and interactive approach. Expert trainers teach and supervise the hands-on process using live patients and different protocols for the extraction, isolation, and application of PRP, adipose- and bone marrow-derived stem cells.
3. Multimedia access. ISSCA provides physicians participating in its onsite training program access to its library of high-resolution, step-by-step procedure videos and ongoing online and telephone support for clinical equipment, inquiries or concerns for the practice’s future use and reference.
• Fellowship in cell therapy and tissue engineering. Recognizing the need for knowledge of stem cell protocols among physicians and healthcare professionals, ISSCA and Stem Cell Training created the Fellowship of Stem Cell Therapy and Tissue Engineering program. The fellowship focuses on stem cell therapies involving the potential replacement of cells or organs that are diseased, injured, infirmed, ailing or aged
In this modular training program, a group of experienced academic scholars involved in stem cell transplantation present a series of topics covering the general principles and practices of stem cell
biology and evidence-based treatments that physicians can apply to optimize the health of their patients. Fellowship course details and objectives include:
• A detailed program offering hands-on experience in stem cell characterization and laboratory applications
• An opportunity to learn cell culture processes including plating, trypsinization, harvesting, and cryopreservation
• Gaining the ability to understand and apply quality control tests including cell count, viability, flow cytometry, endotoxin, mycoplasma, and sterility
• Learning to perform CGMP functions including clean room maintenance, gowning, and environmental monitoring
• Establishing insight on relevant applications of stem cell processing and regulations that apply to a certified facility
• Receiving the tools necessary to implement regulatory and clinical guidelines when setting up a GMP facility
• Providing participants with copies of presentations, procedural protocols, and all forms associated with a GMP facility, as well as case books and full protocols for approximately 30 indications
• Demonstrating the ability to perform clinical procedures including lipoaspirate and bone marrow isolation, and reintroduction of stem cells for various indications
The new ISSCA stem cell training web page also features an informative blog that publishes four new articles in the field of regenerative medicine weekly.
To learn more, visit the ISSCA stem cell training web page, email info@stemcellsgroup.com, or call 305-560-5337.
About ISSCA:
The International Society for Stem Cell Application (ISSCA) is a multidisciplinary community of scientists and physicians, all of whom aspire to treat diseases and lessen human suffering through advances in science, technology and the practice of regenerative medicine. ISSCA serves its members through advancements made in the specialty of regenerative medicine.
The ISSCA’s vision is to take a leadership position in promoting excellence and setting standards in the regenerative medicine fields of publication, research, education, training, and certification.
As a medical specialty, regenerative medicine standards and certifications are essential, which is why ISSCA offers certification training in cities all over the world. The goal is to encourage more physicians to practice regenerative medicine and make it available to benefit patients both nationally and globally. Incorporated under the Republic of Korea as a non-profit entity, ISSCA is focused on promoting excellence and standards in the field of regenerative medicine.
Stem cell training web page launch
- Published in Press Releases
Adimarket announces launch of Cellgenic stem cell protein extract hair restoration product
GSCG affiliate Adimarket announces the launch of the Cellgenic adipose-derived stem cell hair restoration application that maximizes the skin and hair follicles’ own revitalizing capabilities.
MIAMI, June 26, 2018—Global Stem Cells Group (GSCG) affiliate Adimarket announces the launch of the Cellgenic adipose-derived stem cells protein extract (CADPE) hair restoration application product.
CADPE combines refined growth factor proteins extracted from human adipose-derived stem cells in a conditioned media and a unique protein formula, to maximize the revitalizing characteristic of skin and hair follicles.
CADPE increases the reproduction of human follicles’ dermal papilla cells, which consist of a distinct population of specialized fibroblasts important in morphogenesis (differentiation and growth) of the hair follicle structure and control of the hair growth cycle, CADPE works by turning over dying skin cells at twice the frequency of normal skin,
CADPE benefits:
• Use of active proteins derived from human sources eliminates concerns with allergic reactions and skin irritation
• The natural composition of the product’s various growth factors acquired from adipose-derived stem cells can provide exceptional biological activity
• CADPE is multi-functioning, exhibiting anti-oxidation and hair re-growth effects
• Each growth factor has a pharmacological effect, but the combination of growth factors has a synergistic effect that can enhance the pharmacological action of single proteins.
• No special training is needed to learn how to use CADPE
• The CADPE application procedure is shorter than other hair restoration protocols, which can take up to eight hours per patient
• CADPE is supported by scientific evidence
When used by aesthetic practitioners, CADPE provides safe, cutting-edge hair thickening and hair follicle proliferation results that are CTFA-approved, take less application time than other hair restoration processes, and is backed by medical research.
Cellgenic adipose-derived stem cells protein extract can be purchased online at the Adimarket online store. For more information, visit the Adimarket website, email info@stemcellsgroup.com, or call 305-560-5337.
About Adimarket: Adimarket, Inc., a division of the Global Stem Cells Group, is a one-stop, cost-competitive online marketplace for quality regenerative medicine equipment and supplies for physicians and healthcare professionals.
Adimarket, Inc., a division of the Global Stem Cells Group, is a one-stop, cost-competitive online marketplace for quality regenerative medicine equipment and supplies for physicians and healthcare professionals.
Adimarket was founded to provide practitioners the tools they need to practice regenerative medicine in a medical office setting. Motivated by a firm belief in the impact stem cell medicine can have when dispensed in a doctor’s office, Adimarket provides physicians with the tools they need to provide patients with cutting-edge treatments.
About Global Stem Cells Group:
Global Stem Cells Group (GSCG) is a worldwide network that combines  seven major medical corporations. Each corporation is focused on furthering scientific and technological advancements in cutting-edge stem cell research, development, treatment, and training. The united efforts of GSCG’s affiliate companies provide medical practitioners with a one-stop epicenter for stem cell solutions that adhere to the highest medical standards.
seven major medical corporations. Each corporation is focused on furthering scientific and technological advancements in cutting-edge stem cell research, development, treatment, and training. The united efforts of GSCG’s affiliate companies provide medical practitioners with a one-stop epicenter for stem cell solutions that adhere to the highest medical standards.
Global stem cell’s mission is to be the largest recognized stem cell and regenerative medicine network in the world.
CADPE stem cell hair restoration application
- Published in Press Releases
Global Stem Cells Group to sponsor the American Association of Stem Cell Physicians summit in Miami
Global Stem Cells Group will sponsor the American Association of Stem Cell Physicians summit in Miami August 10 – 12, 2018.
MIAMI, June 18, 2018—Global Stem Cells Group (GSCG), a world leader in regenerative medicine, announces its sponsorship of the American Association of Stem Cell Physicians (AAOSCP) summit in Miami, August 10-12, 2018. The conference will offer three days of educational, workshop, and networking events with physicians and researchers from around the world in the fields of stem cell and regenerative medicine. Attending physicians can earn up to 24 CME credits.
Summit attendees will have the opportunity to learn about Adimarket, GSCG’S fast-growing online store for regenerative medicine practitioners to conveniently purchase products, supplies, and equipment that meets their specific clinical needs.
GSCG affiliate Stem Cell Training, Inc. (SCT) will also be on hand to provide qualified Summit attendees with personalized, hands-on regenerative medicine training course at the summit, which will be conducted by expert instructors who guide qualified physicians through the intensive, two-day certification program. Benefits of this stem cell training course include:
- Stem Cell Training’s state-of-the-art, one-on-one regenerative medicine training on the latest stem cell procedures and protocols to use immediately in private clinical practice
- Access to SCT’s online resources, personalized theoretical information, and hands-on training with live patients, as well as ongoing support for each participating physician’s clinical practice
- Stem cell applications and protocols demonstrated by an SCT faculty member with extensive experience in laboratory and clinical practices
Physicians and businesses professionals in the field of regenerative medicine, the AAOSCP summit offers an outstanding opportunity to connect with others in the industry, explore, learn, and make connections while learning about the latest groundbreaking treatments and technologies. Medical students and those seeking careers on the business side of the regenerative medicine market can take advantage of the valuable opportunity the summit offers to network with potential employers while learning about groundbreaking treatments and therapies.
Since 2014, Global Stem Cells Group has worked with some of the most prestigious regenerative medicine practitioners in the U.S., South America, and Asia as it focuses on growing its services throughout the global community. Stem cell therapies continue to revolutionize the healthcare industry while offering new hope for sufferers of chronic, debilitating conditions.
For more information and to register for the summit, visit the AAOSCP website, email info@stemcellsgroup.com, or call 305-560-5337.
About Global Stem Cells Group
Global Stem Cells Group (GSCG) is a worldwide network that combines seven major medical corporations. Each corporation is focused on furthering scientific and technological advancements in cutting-edge stem cell research, development, treatment, and training. The united efforts of GSCG’s affiliate companies provide medical practitioners with a one-stop epicenter for stem cell solutions that adhere to the highest medical standards.
Global stem cell’s mission is to be the largest recognized stem cell and regenerative medicine network in the world.
American Association of Stem Cell Physicians summit
- Published in Press Releases

![yt-e1548699872543[1]](https://stemcellcenter.net/wp-content/uploads/2019/11/yt-e15486998725431-460x260_c.png)
![mapa-de-europa-y-africa-[1]](https://stemcellcenter.net/wp-content/uploads/2019/11/mapa-de-europa-y-africa-1-460x260_c.jpg)
![GSCG_VLOG[1]](https://stemcellcenter.net/wp-content/uploads/2019/11/GSCG_VLOG1-460x260_c.png)
![surgical[1]](https://stemcellcenter.net/wp-content/uploads/2019/11/surgical1-460x260_c.jpg)
![CELL_THERAPY2[1]](https://stemcellcenter.net/wp-content/uploads/2019/11/CELL_THERAPY21-460x260_c.png)
![STEM-CELL-TRAINING[1]](https://stemcellcenter.net/wp-content/uploads/2019/11/STEM-CELL-TRAINING1-460x260_c.png)
![STEM_CELL_TRAINING_WEBSITE_LAUNCH[1]](https://stemcellcenter.net/wp-content/uploads/2019/11/STEM_CELL_TRAINING_WEBSITE_LAUNCH1-460x260_c.png)
![cadpe[1]](https://stemcellcenter.net/wp-content/uploads/2019/11/cadpe1-460x260_c.png)