Global Stem Cells Group Launches Two Stem Cell Treatment Centers in Arica and Iquique, Chile

Global Stem Cells Group announces the launch of two new stem cell treatment clinics in the cities of Arica and Iquique in northern Chile. The facilities are part of the international biotech company’s expanding presence in Latin America.
MIAMI, May 31, 2016—Global Stem Cells Group, a leading international biotechnology company, announces the launch of operations at two new GSCG clinics in the cities of Arica and Iquique in northern Chile. The facilities are part of GSCG’s expanding presence in Latin America.
Both the Arica and Iquique clinics offer the most advanced protocols and techniques in stem cell medicine to patients from around the world.

The clinics are headed by stem cell specialists Victor Perez, M.D., and Duval Aguirre, M.D., and will offer treatments in chronic degenerative conditions, Type 2 diabetes, COPD, traumatology and sports medicine.
Global Stem Cells Group, has been expanding its clinical presence throughout Latin America and worldwide by partnering with qualified physicians experienced in stem cell therapies to open new clinics. The new Arica and Iquique clinics are certified for the medical tourism market.
Global Stem Cells Group is committed to the highest standards in service and technology, expert and compassionate care, and a philosophy of exceeding the expectations of their international patients.
For more information, visit the Global Stem Cells Group website, Email bnovas@stemcellsgroup.com, or call 305-560-5337.
About the Global Stem Cells Group:

Global Stem Cells Group, Inc. is the parent company of six wholly owned operating companies dedicated entirely to stem cell research, training, products and solutions. Founded in 2012, the company combines dedicated researchers, physician and patient educators and solution providers with the shared goal of meeting the growing worldwide need for leading edge stem cell treatments and solutions.
With a singular focus on this exciting new area of medical research, Global Stem Cells Group and its subsidiaries are uniquely positioned to become global leaders in cellular medicine.
Global Stem Cells Groups corporate mission is to make the promise of stem cell medicine a reality for patients around the world. With each of GSCGs six operating companies focused on a separate research-based mission, the result is a global network of state-of-the-art stem cell treatments.
To view this press release live online, click here
###
- Published in Press Releases
Duncan Ross, Ph.D. Joins Global Stem Cells Group Advisory Board
Molecular biologist, immunologist and researcher Duncan Ross, Ph.D., founder of Kimera Labs, has joined the Global Stem Cells Group Advisory Board faculty.
Global Stem Cells Group CEO Benito Novas announced that Duncan Ross, Ph.D. has joined the GSCG Advisory Board. Ross is the founder of GSCG affiliate Kimera Labs in Miami.
Ross is the founder of GSCG affiliate Kimera Labs in Miami.
In 2004, Ross received his Ph.D. in Immunology from the University of Miami, where he studied hematopoietic stem cell transplantation for hematologic disorders and the suppression of graft vs. host disease (GVHD) under UM Professor Robert Levy Ph.D. Ross was motivated to study in this field after witnessing his father’s experience suffering through unsuccessful treatments for Acute Myeloid Leukemia.
Since launching his career in 2004, Ross has investigated various methods of immune suppression, from regulatory T cells to post transplant cyclophosphamide, in collaboration with the Leo Luznik Transplant Lab at Johns Hopkins University in Baltimore, Maryland. The collaboration led to multiple publications in peer-reviewed journals, including “Blood” and “Biology of Blood and Marrow Transplantation 1,2.”
 Ross’s interest in the use of mesenchymal stem cells (MSCs) for immune suppression stemmed from this work and the work of others in the use of MSCs to treat GVHD.
Ross’s interest in the use of mesenchymal stem cells (MSCs) for immune suppression stemmed from this work and the work of others in the use of MSCs to treat GVHD.
In 2008, Ross founded Kimera Labs in Miami, currently focused on the use of MSCs for the suppression of various immune mediated pathologies and regenerative medicine in the US, Latin America, and the Bahamas.
Ross recently obtained Institutional Review Board (IRB) approval to perform a patient sponsored clinical trial for chronic obstructive pulmonary disorder (COPD) using mesenchymal stem cells purified from fat, through the Kimera Society. Kimera Labs in association with Global Stem Cells Group plans to apply the same approach to diabetes and other autoimmune diseases.
Ross joins a group of esteemed stem cell researchers and physicians on the Global Stem Cells Group Advisory Board, who contribute their expertise and provide strategic advice for the management of the international biotech company.
To learn more, visit the Global Stem Cells Group website, email bnovas(at)stemcellsgroup(dot)com, Or call +1 305 560 5337.
About Global Stem Cell Group:
Global Stem Cells Group, Inc. is the parent company of six wholly owned operating companies dedicated entirely to stem cell research, training, products and solutions. Founded in 2012, the company combines dedicated researchers, physician and patient educators and solution providers with the shared goal of meeting the growing worldwide need for leading edge stem cell treatments and solutions. With a singular focus on this exciting new area of medical research, Global Stem Cells Group and its subsidiaries are uniquely positioned to become global leaders in cellular medicine.
Global Stem Cells Group’s corporate mission is to make the promise of stem cell medicine a reality for patients around the world. With each of GSCG’s six operating companies focused on a separate research-based mission, the result is a global network of state-of-the-art stem cell treatments.
###
To view this press release live online, click here
- Published in Press Releases
Global Stem Cells Group and University of Santiago Biotech Lab Endorse Asia-Pacific Symposium
Global Stem Cells Group and the University of Santiago, Chile have endorsed an Asian-Pacific alliance for a regenerative medicine and stem cell symposium July 1-2 at the university’s Santiago campus and other stem cell protocol management initiatives. Through the alliance, the two organizations established a working agenda for collaborative initiatives in stem cell and regenerative medicine research and development for 2016 – 2020.
Global Stem Cells Group and the University of Santiago Biotechnology Lab have announced a mutual endorsement of an Asia-Pacific Symposium as other research and development initiatives for potential stem cell protocol management for 2016 – 2020.
 In 2015, University of Santiago officials and top Global Stem Cells Group executives began meeting to establish a working agenda and foster initiatives to promote stem cell research and development as a collaborative effort.
In 2015, University of Santiago officials and top Global Stem Cells Group executives began meeting to establish a working agenda and foster initiatives to promote stem cell research and development as a collaborative effort.
Professor Alejandra Moenen, Ph.D., who heads the University of Santiago’s Biochemistry and Molecular Biology Department, and a team of Ph.D.s from the university will join Global Stem Cells Group for their first joint venture, an Asia-Pacific Symposium on stem cell and regenerative medicine July 1-2, 2016. Moenen is an internationally prominent researcher whose work in biological research has been published in 50 major scientific journals worldwide.
The symposium will focus on regenerative medicine and stem cell applications to anti-aging and aesthetic medicine. University of Santiago faculty will lead the symposium, which will host qualified academic and medical groups from around the world who will present their scientific papers.
Global Stem Cells Group and the University of Santiago’s Biotechnology Department decided to join forces and create a collaborative agenda based on the synergy between the two organizations.
“Chile is a country where we have first world science, without being part of developed countries,” says Moenen. “Today  we are proud to start an alliance through which we can work hand in hand with Global Stem Cells Group and its international network, which has been able to harness science to improve the quality of life for people. “
we are proud to start an alliance through which we can work hand in hand with Global Stem Cells Group and its international network, which has been able to harness science to improve the quality of life for people. “
Enrique Testart, M.D., Chief Medical Officer of Global Stem Cells Group, says he was honored to learn that a Chilean
University had initiated this new approach to collaborating with GSCG, which he believes will offer unparalleled opportunities for exchange, relationships with other institutions, and all the technology that Global Stem Cells Group can offer for studies and analysis in the area of regenerative medicine.
“A range of criteria and this innovative university are what stand out in this framework agreement,” Testart says. “It places us above any attempt to trivialize the issue.
Enrique Testart, M.D.
“Stem cells are not a fad, there are those who have been working for two decades in this field, and therefore the union between this esteemed university and this young and talented biotech company is good news for the country, for the world and for science—everyone should applaud.”
A meeting to confirm the Asia-Pacific Symposium alliance was attended by Kevin Maisey, Ph.D., and Jorge LaPorte, Ph.D., both representing the Biology and Biochemistry Department of the University of Santiago. University Dean Silvia Ferrada Vergara has validated the agreement, which will be announces at the Asia-Pacific Conference in July.
For more information, visit the Global Stem Cells Group website, email bnovas(at)stemcellsgroup(dot)com, or call +1 305 560 5337.
About Global Stem Cell Group:
Global Stem Cells Group, Inc. is the parent company of six wholly owned operating companies dedicated entirely to stem cell research, training, products and solutions. Founded in 2012, the company combines dedicated researchers, physician and patient educators and solution providers with the shared goal of meeting the growing worldwide need for leading edge stem cell treatments and solutions. With a singular focus on this exciting new area of medical research, Global Stem Cells Group and its subsidiaries are uniquely positioned to become global leaders in cellular medicine.
Global Stem Cells Group’s corporate mission is to make the promise of stem cell medicine a reality for patients around the world. With each of GSCG’s six operating companies focused on a separate research-based mission, the result is a global network of state-of-the-art stem cell treatments.
About the University of Santiago:
Celebrating the 166th anniversary of its founding in 2016, the University of Santiago is one of the oldest and most traditional institutions of higher education in Chile. Offering 66 comprehensive undergraduate programs to more than 18,000 students, the university has seven faculties representing departments of Engineering, Humanities, Science, Business and Economics, Chemistry and Biology, Medical Sciences and Technology. The university us moving toward a new era of implementing improved and advanced master’s degree and doctoral degree programs, in addition to the numerous courses and postgraduate programs already in place in a variety of academic and research disciplines.
Since Chile’s 1981 higher education reform, the University of Santiago has concentrated its activities in the metropolitan area, with a particular focus on teaching, research and extension, carried out on the 34-hectare (84 acre) campus in the City of Santiago.
The University of Santiago is known for its participation in national and international projects and the contributions of its scholars to various fields of knowledge. A singular effort has been placed on linking the work of university researchers, who have a close relationship with the socio-economic needs of the country, to improve public health conditions in the country. The University of Santiago is one of Chile’s four Universities noted for successful fundraising efforts to support research and development.
###
To view this press release online, click here
- Published in Press Releases
Global Stem Cells Group Advisory Board Member Duncan Ross, Ph.D., to Speak at Santiago, Chile Symposium
Global Stem Cells Group Advisory Board member Duncan Ross, Ph.D., founder of Kimera Research Labs, will be the keynote speaker at the Global Stem Cells Group Symposium in Santiago, Chile July 1-2, 2016.
 MIAMI, April 26, 2016–Global Stem Cells Group has announced that affiliate Kimera Research Labs founder Duncan Ross, Ph.D., a GSCG Advisory Board member, will be the keynote speaker at the Asia-Pacific Symposium in Santiago Chile, July 1-2, 2016. The abstract for Ross’s lecture will be, “The mechanism of action of stem cells in regenerative medicine is increasingly being understood to be effected through paracrine factors. Central to the question of when and how to treat an individual disease is where and for what duration a transplanted cell will persist to generate these factors.”
MIAMI, April 26, 2016–Global Stem Cells Group has announced that affiliate Kimera Research Labs founder Duncan Ross, Ph.D., a GSCG Advisory Board member, will be the keynote speaker at the Asia-Pacific Symposium in Santiago Chile, July 1-2, 2016. The abstract for Ross’s lecture will be, “The mechanism of action of stem cells in regenerative medicine is increasingly being understood to be effected through paracrine factors. Central to the question of when and how to treat an individual disease is where and for what duration a transplanted cell will persist to generate these factors.”
In the absence of a robust ability to track cell persistence in humans, Dr. Ross will present current research in murine hematopoietic and mesenchymal stem cell transplantation with support from human transplant results.

Duncan Ross, Ph.D.
The symposium will be co-sponsored by Global Stem Cells Group and the University of Santiago’s Biochemistry and Molecular Biology Department, and will focus on regenerative medicine and stem cell applications to anti-aging and aesthetic medicine. University of Santiago faculty will lead the symposium, which will host qualified academic and medical groups from around the world who will present their scientific papers.
The symposium is the first joint endeavor between Global Stem Cells Group and the University of Santiago since establishing an alliance recently, and which will be announced at the Asia-Pacific Symposium. It also marks Ross’s first appearance as a member of the Global Stem Cells Group Advisory Board.
Ross received a Ph.D. in Immunology from the University of Miami and specializes in research, mesenchymal stem cell applications, hematopoietic stem cell transplantation for hematologic disorders, the suppression of graft vs. host disease, and var
ious methods of immune suppression.
Global Stem Cells Group and Kimera Labs share a commitment to research and development, and providing stem cell treatments to patients in clinical settings worldwide.
To learn more, visit the Global Stem Cells Group website, email bnovas(at)stemcellsgroup(dot)com, or call +1 305 560 5337.
About Global Stem Cell Group:
Global Stem Cells Group, Inc. is the parent company of six wholly owned operating companies dedicated entirely to stem cell research, training, products and solutions. Founded in 2012, the company combines dedicated researchers, physician and patient educators and solution providers with the shared goal of meeting the growing worldwide need for leading edge stem cell treatments and solutions. With a singular focus on this exciting new area of medical research, Global Stem Cells Group and its subsidiaries are uniquely positioned to become global leaders in cellular medicine.
Global Stem Cells Group’s corporate mission is to make the promise of stem cell medicine a reality for patients around the world. With each of GSCG’s six operating companies focused on a separate research-based mission, the result is a global network of state-of-the-art stem cell treatments.
About Kimera Labs:
Kimera Labs is currently focused on the use of mesenchymal stem cells (MSCs) for the suppression of various immune mediated pathologies and regenerative medicine in the US, Latin America, and the Bahamas. Founder Duncan Ross, Ph.D., is an immunologist and researcher who has studied hematopoietic stem cell transplantation for hematologic disorders, the suppression of graft vs. host disease, and various methods of immune suppression.
Kimera Labs provides patients access to stem cell treatment in the U.S. according to U.S. laws. In order to provide the greatest benefit to patients, Ross frequently travels to treat patients in Central and South America where specialists are available in a different regulatory environment.
###
To view this press release online, click here
- Published in Press Releases
Global Stem Cells Group and Kimera Labs Announce Autologous Stem Cell Research Alliance
Global Stem Cells Group and Kimera Research Labs have announced an alliance to conduct scientific research on highly manipulated cells and culture expansion, and cryopreservation of autologous stem cells.
MIAMI, April 26, 2016–Global Stem Cells Group and Kimera Research Labs have announced an alliance to conduct  scientific research on highly manipulated stem cells and culture expansion, and cryopreservation of autologous stem cells. The collaboration will open new opportunities for GSCG to increase its participation in scientific research and development of new stem cell protocols and treatments for a number of conditions.
scientific research on highly manipulated stem cells and culture expansion, and cryopreservation of autologous stem cells. The collaboration will open new opportunities for GSCG to increase its participation in scientific research and development of new stem cell protocols and treatments for a number of conditions.
The manipulation of stem cells involves the ability to deliver molecules into adherent cells without disrupting differentiation, a process biotechnology researchers need in order to advance both fundamental knowledge and the state-of-the-art in stem cell research. Differentiation is the process by which an unspecialized cell, such as a stem cell, becomes specialized into one of the many cells in the body. During differentiation, certain genes become activated and other genes become inactivated in an painstakingly regulated manner. As a result, a differentiated cell develops specific structures and performs certain functions that ultimately allows it to replace damaged or dead cells. In the laboratory, a stem cell can be manipulated to become specialized or partially specialized cell types, such as heart muscle, nerve, or pancreatic cells.
“Non-destructive manipulation of stem cells in the correct environment is key to enabling technology needed within the biology and medical research communities,” says Benito Novas, CEO of Global Stem Cells Group. “To realize the promise of stem cell-based therapies to treat injuries and diseases, scientists must be able to manipulate stem cells so that they possess the necessary characteristics for successful differentiation, transplantation, and engraftment.”
To bring successful new treatments to the clinic, scientists need to control certain steps for stem cells to be useful for transplant purposes. Researchers are constantly discovering new ways to manipulate stem cells to be reproducibly made to:
- Replicate extensively and generate sufficient quantities of cells for making tissue.
- Differentiate into the desired cell type(s).
- Survive in the recipient after transplant.
- Integrate into the surrounding tissue after transplant.
- Function appropriately for the duration of the recipient’s life.
- Avoid harming the recipient in any way.
Scientists are also experimenting with different research strategies to generate tissue without the concern of immune rejection.
 Research on cryopreservation of autologous stem cells is necessary for cell bank procedures in which stem cell expansion and use are not immediately needed. Cryopreservation allows for the long-term storage of hematopoietic stem cells (HSCs) and is the preferred storage technique for virtually all components intended for autologous HSC transplantation.
Research on cryopreservation of autologous stem cells is necessary for cell bank procedures in which stem cell expansion and use are not immediately needed. Cryopreservation allows for the long-term storage of hematopoietic stem cells (HSCs) and is the preferred storage technique for virtually all components intended for autologous HSC transplantation.
Cryopreservation allows the administration of multiple-day transplant conditioning regimens as well as elective storage for patients to receive transplants at a subsequent point in a course of treatment, and offers patients the opportunity to benefit from multidose protocols.
Global Stem Cells Group and Kimera Labs share a commitment to research, develop and provide stem cell treatments to patients worldwide in a clinical setting.
To learn more, visit the Global Stem Cells Group website, email bnovas(at)stemcellsgroup(dot)com, or call +1 305 560 5337.
About Global Stem Cell Group:
Global Stem Cells Group, Inc. is the parent company of six wholly owned operating companies dedicated entirely to stem cell research, training, products and solutions. Founded in 2012, the company combines dedicated researchers, physician and patient educators and solution providers with the shared goal of meeting the growing worldwide need for leading edge stem cell treatments and solutions. With a singular focus on this exciting new area of medical research, Global Stem Cells Group and its subsidiaries are uniquely positioned to become global leaders in cellular medicine.
Global Stem Cells Group’s corporate mission is to make the promise of stem cell medicine a reality for patients around the world. With each of GSCG’s six operating companies focused on a separate research-based mission, the result is a global network of state-of-the-art stem cell treatments.
About Kimera Labs:
Kimera Labs is currently focused on the use of mesenchymal stem cells (MSCs) for the suppression of various immune mediated pathologies and regenerative medicine in the US, Latin America, and the Bahamas. Founder Duncan Ross, Ph.D., is an immunologist and researcher who has studied hematopoietic stem cell transplantation for hematologic disorders, the suppression of graft vs. host disease, and various methods of immune suppression.
Kimera Labs provides patients access to stem cell treatment in the U.S. according to U.S. laws. In order to provide the greatest benefit to patients, Ross frequently travels to treat patients in Central and South America where specialists are available in a different regulatory environment.
###
To view this press release live on line, click here
- Published in Press Releases
Global Stem Cells Group Plans Bone Marrow Clinical Trials for Knee Osteoarthritis
Global Stem Cells Group has announced plans to hold clinical trials, pending IRB approval, for bone marrow stem cell treatments targeting knee osteoarthritis. The trials will be held in five GSCG facilities in the U.S. and South America, with 25 patients accepted for each location.
MIAMI, March 31, 2016—Pending Institutional Review Board (IRB) approval, Global Stem Cells Group, Inc. has announced plans to conduct a multi-center, placebo controlled clinical trial to measure the safety and effectiveness of the intra-articular application of freshly isolated bone marrow stem cells for the treatment of osteoarthritis.
The clinical trials, which will begin July 1, 2016 and run for one year, will be held in Global Stem Cell Group facilities in Buenos Aires, Argentina; Bogota, Colombia; Quito, Ecuador; Miami, Florida and Topeka, Kansas. Each center will accept 25 patients per clinical trial, and patients will receive a bone marrow stem cell injection in one knee and a placebo in the other knee..
 The trials are designed to investigate the possible beneficial effect of freshly harvested bone marrow stem cell applications on knee osteoarthritis patients in the control group. Patients will receive standard treatment of bone marrow stem cells intravenously, and will be monitored and assessed for any changes in clinical condition.
The trials are designed to investigate the possible beneficial effect of freshly harvested bone marrow stem cell applications on knee osteoarthritis patients in the control group. Patients will receive standard treatment of bone marrow stem cells intravenously, and will be monitored and assessed for any changes in clinical condition.
Knee osteoarthritis is a chronic, progressive condition affecting an increasing number of people, especially the elderly and obese. It is characterized by degeneration of the cartilage—the natural cushioning between joints inside the knee.
The condition is the result of the wearing away of cartilage. When this happens, the bones of the joints rub more closely against one another with less of the shock-absorbing benefits of cartilage, resulting in pain, swelling, stiffness and a decreased ability to move.
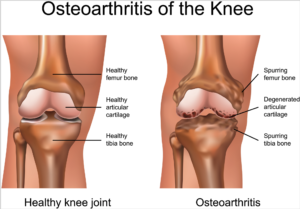 According to the Centers for Disease Control (CDC), knee osteoarthritis will affect 67 million people in the United States by 2030. While conventional treatments like physiotherapy or drugs offer temporary relief of clinical symptoms, total knee replacement is the closest treatment available for permanent relief, which requires invasive surgery, comes at a high cost and is not always successful. The latest advances in stem cell therapies for knee osteoarthritis are designed to restore cartilage function in the knee.
According to the Centers for Disease Control (CDC), knee osteoarthritis will affect 67 million people in the United States by 2030. While conventional treatments like physiotherapy or drugs offer temporary relief of clinical symptoms, total knee replacement is the closest treatment available for permanent relief, which requires invasive surgery, comes at a high cost and is not always successful. The latest advances in stem cell therapies for knee osteoarthritis are designed to restore cartilage function in the knee.
Global Stem Cells Group offers the most advanced protocols and techniques in cellular medicine from around the world.
Details of the protocol and eligibility criteria will be released upon IRB approval.
For more information on Global Stems Cell Group, visit the Global Stem Cells Group website, email bnovas(at)stemcellsgroup(dot)com, or call +1 305 560 5337.
About Global Stem Cells Group:
Global Stem Cells Group, Inc, is the parent company of six wholly owned operating companies dedicated entirely to stem cell research, training, products and solutions. Founded in 2012, the company combines dedicated researchers, physician and patient educators and solution providers with the shared goal of meeting the growing worldwide need for leading edge stem cell treatments and solutions. With a singular focus on this exciting new area of medical research, Global Stem Cells Group and its subsidiaries are uniquely positioned to become global leaders in cellular medicine.
Global Stem Cells Group’s corporate mission is to make the promise of stem cell medicine a reality for patients around the world. With each of GSCG’s six operating companies focused on a separate research-based mission, the result is a global network of state-of-the-art stem cell treatments.
To view this press release live online, click here
###
- Published in Press Releases
Global Stem Cells Group Announces Manufacturing Phase of Progenikine™ SVF Closed System
Global Stem Cells Group has begun the manufacturing phase of Progenikine™, a new SVF closed system kit utilizing EmCyte technology, containing all the elements necessary to process adipose tissue and obtain stromal vascular fraction in a sterile environment.
MIAMI, March 31, 2016—Global Stem Cells Group, Inc. has announced that Progenikine™, its new and approved SVF closed system kit using EmCyte technology, is in the manufacturing phase and will be available to physicians in July 2016. The Progenikine kit contains all the elements necessary to process adipose tissue and obtain stromal vascular fraction (SVF) in a closed environment.
Adipose derived stem cells (ASCs) are used by physicians for a variety of indications. Most commonly, ASCs are  isolated at the point of care from lipoaspirate (derived from liposuction) tissue as the stromal vascular fraction (SVF), harvested from the patient and immediately administered to the patient as an injection, or used to enrich fat grafts. Isolation of ASCs from adipose tissue is a relatively simple process performed routinely in cell biology laboratories, but isolation at the point of care for immediate clinical administration requires special methodology to prevent contamination, ensure integrity of the clinical procedure, and comply with regulatory requirements.
isolated at the point of care from lipoaspirate (derived from liposuction) tissue as the stromal vascular fraction (SVF), harvested from the patient and immediately administered to the patient as an injection, or used to enrich fat grafts. Isolation of ASCs from adipose tissue is a relatively simple process performed routinely in cell biology laboratories, but isolation at the point of care for immediate clinical administration requires special methodology to prevent contamination, ensure integrity of the clinical procedure, and comply with regulatory requirements.
Developed in conjunction with Patrick Pennie, Emcyte CEO, and and Maritza Novas Director of Research and Development for Global Stem Cells Group, Progenikine  fuses elements from Emcyte systems with the Global Stem cells Group SVF protocols.The kit can provide a low cost, rapid and simple alternative to traditional methods of isolating ASCs, particularly when smaller quantities are needed.
fuses elements from Emcyte systems with the Global Stem cells Group SVF protocols.The kit can provide a low cost, rapid and simple alternative to traditional methods of isolating ASCs, particularly when smaller quantities are needed.
“The Progenikine kit is the newest product designed to help Global Stem Cells Group’s mission to provide accessible products to our member clients, ensuring that more patients will be able to gain access to stem cell therapies,” says Benito Novas, GSCG CEO.
For more information on Global Stems Cell Group, visit the Global Stem Cells Group website,email bnovas(at)stemcellsgroup(dot)com, or call +1 305 560 5337.
About Global Stem Cells Group:
Global Stem Cells Group, Inc. is the parent company of six wholly owned operating companies dedicated entirely to stem cell research, training, products and solutions. Founded in 2012, the company combines dedicated researchers, physician and patient educators and solution providers with the shared goal of meeting the growing worldwide need for leading edge stem cell treatments and solutions. With a singular focus on this exciting new area of medical research, Global Stem Cells Group and its subsidiaries are uniquely positioned to become global leaders in cellular medicine.
Global Stem Cells Group’s corporate mission is to make the promise of stem cell medicine a reality for patients around the world. With each of GSCG’s six operating companies focused on a separate research-based mission, the result is a global network of state-of-the-art stem cell treatments.
About Emcyte:
Fort Myers, Florida-based EmCyte Corporation is a leader in autologous cellular biologics with the GenesisCS Component Concentrating Systems. These systems provide patients with the best opportunity for rapid recovery and provide practitioners with the most advanced clinical point of care experience. EmCyte systems are developed to meet every clinical requirement, giving the physician better clinical choices. EmCyte devices have been independently reviewed and show to produce buffycoat concentrations of 6x to greater than 10x baseline in 7mLs, with yields ranging from 70 percent to greater than 90 percent
EmCyte technology allows for the safe extraction of concentrated platelets and other regenerative cell types from the patient’s own blood. These cells are then re-suspended in a small volume of the patient’s blood plasma and then applied to the treatment site.
###
To view this press release live online, click here
- Published in Press Releases
Global Stem Cells group to Launch New Clinical Site in Quito, Ecuador with an Inaugural Conference and Stem Cell Training Event
Global Stem Cells Group has announced plans to inaugurate its new stem cell center in Quito, Ecuador with an inaugural conference and stem cell training event Feb. 24-March 6. The new facility will offer the latest stem cell therapies to treat a variety of patient needs.
MIAMI, Feb. 22, 2016—Global Stem Cells Group, has announced an inaugural conference and stem cell training event to launch  its new stem cell treatment in Quito, Ecuador. Feb. 24-March 6, 2016 launch of a new stem cell treatment center in Quito, Ecuador. The new facility will provide advanced protocols and state-of-the-art techniques in cellular medicine to patients from around the world.
its new stem cell treatment in Quito, Ecuador. Feb. 24-March 6, 2016 launch of a new stem cell treatment center in Quito, Ecuador. The new facility will provide advanced protocols and state-of-the-art techniques in cellular medicine to patients from around the world.
Global Stem Cells Group CEO Benito Novas will host the event, which will begin with a stem cell conference featuring a renowned group of stem cell experts who will be featured speakers, including Global Stem Cells Group Chief Operating Officer Kipp Van Camp, D.O., Joseph Purita, M.D., and Pablo Cornejo, M.D. Purita, who heads GSCG’s Stem Cell Training, and Cornejo will perform a stem cell treatment on a high level Ecuadorian government official to address an orthopedic condition. The official cannot be named due to medical privacy standards.

Joseph Purita, M.D.
The opening of the Quito stem cell clinic—the first in Ecuador—is part of Global Stem Cells Group’s expanding presence in Latin America.
“We’re excited to launch the Quito Global Stem Cells Group clinic and establish our services in Ecuador,” says Novas. “The Quito clinical staff of highly qualified and regarded physicians and medical professionals is enthusiastic about their roles as pioneers in bringing stem cell therapies to the country.”

Kipp Van Camp, D.O.
The Quito event will be held at the Hotel Hilton Quito.
Global Stem Cells Group provides stem cell treatments for a variety of conditions and diseases including arthritis, autism, chronic obstructive pulmonary disease (COPD), diabetes, multiple sclerosis, injuries and more at various facilities worldwide. The new facility in Quito will have an

Pablo Cornejo, M.D.
Global Stem Cells Group’s Quito clinic is certified for the medical tourism market, and staff physicians are board-certified or board-eligible. GSCG clinics provide services in more than 10 specialties, attracting patients from the United States and around the world.
The Global Stem Cells Group is committed to providing the highest of standards of services and technology, expert and compassionate care, and a philosophy of exceeding the expectations of their international patients.
For more information, visit the Global Stem Cells Group website, email bnovas(at)stemcellsgroup(dot)com, or call +1 305 560 5337.
About the Global Stem Cell Group:
Global Stem Cells Group, Inc. is the parent company of six wholly owned operating companies dedicated entirely to stem cell research, training, products and solutions. Founded in 2012, the company combines dedicated researchers, physician and patient educators and solution providers with the shared goal of meeting the growing worldwide need for leading edge stem cell treatments and solutions. With a singular focus on this exciting new area of medical research, Global Stem Cells Group and its subsidiaries are uniquely positioned to become global leaders in cellular medicine.
Global Stem Cells Group’s corporate mission is to make the promise of stem cell medicine a reality for patients around the world. With each of GSCG’s six operating companies focused on a separate research-based mission, the result is a global network of state-of-the-art stem cell treatments.
To view this press release live online, click here
###
- Published in Press Releases
Global Stem Cells Group Announces New Clinic Opening in Quito, Ecuador
Global Stem Cells Group has announced the opening of a new stem cell clinic in Quito, Ecuador. The new facility will offer the latest stem cell and regenerative medicine treatments for orthopedic and trauma applications.
MIAMI, Feb. 11, 2016—Global Stem Cells Group, has announced the opening of its new stem cell treatment clinic in Quito, Ecuador. The new facility will provide advanced protocols and state-of-the-art techniques in cellular medicine, focusing on orthopedic and trauma applications to patients from around the world.
Ecuador. The new facility will provide advanced protocols and state-of-the-art techniques in cellular medicine, focusing on orthopedic and trauma applications to patients from around the world.

Pablo Cornejo, MD
The new GSCG clinic is headed by four prominent Ecuadorian physicians, including Pablo Ramos, M.D. an orthopedic specialist and attending physician at Metropolitan Hospital in Quito; Pablo Cornejo, M.D., a specialist in orthopedic and trauma surgery at Metropolitan Hospital, Quito; Luis Ernesto Mantilla, M.D., a specialist in trauma and knee Surgery,at Metropolitan Hospital, Quito, and Pablo Ramos, M.D., a specialist in trauma surgery and arthroscopy at Metropolitan Hospital, Quito.
The opening of the Quito stem cell clinic marks Global Stem Cells Group’s first facility in Ecuador, and is part of GSCG’s expanding presence in Latin America.
[su_spacer size=”10″]

Luis Ernesto Mantilla, MD
Global Stem Cells Group has been expanding its clinical presence worldwide by partnering with qualified physicians experienced in stem cell therapies to open new clinics, licensed and developed under the GSCG banner.
Global Stem Cells Group provides stem cell treatments for a variety of conditions and diseases including arthritis, autism, chronic obstructive pulmonary disease (COPD), diabetes, multiple sclerosis, injuries and more at various facilities worldwide. The new facility in Quito will have an international staff that is experienced in administering the leading cellular therapies available.
Global Stem Cells Group’s Quito clinic is certified for the medical tourism market, and staff physicians are board-certified or board-eligible. GSCG clinics provide services in more than 10 specialties, attracting patients from the United States and around the world.

Pablo Ramos, M.D.
For more information, visit the Global Stem Cells Group website, email bnovas(at)stemcellsgroup(dot)com, or call +1 305 560 5337.
About the Global Stem Cell Group:
Global Stem Cells Group, Inc. is the parent company of six wholly owned operating companies dedicated entirely to stem cell research, training, products and solutions. Founded in 2012, the company combines dedicated researchers, physician and patient educators and solution providers with the shared goal of meeting the growing worldwide need for leading edge stem cell treatments and solutions. With a singular focus on this exciting new area of medical research, Global Stem Cells Group and its subsidiaries are uniquely positioned to become global leaders in cellular medicine.
Global Stem Cells Group’s corporate mission is to make the promise of stem cell medicine a reality for patients around the world. With each of GSCG’s six operating companies focused on a separate research-based mission, the result is a global network of state-of-the-art stem cell treatments.
To view this press release live online, click here
###
- Published in Press Releases
Global Stem Cells Group Named Authorized Distributor of GMTN Exosome Injection and Other Biological Products
Global Stem Cells Group, a world leader in regenerative medicine, has announced a new agreement with  Bankok,Thailand-based Global Stem Cells Network (GSCN) to distribute exosome injection and other biological products to stem cell researchers and phsyicians in 15 Latin American countries, including Mexico, Costa Rica, Dominican Republic, Colombia, Argentina, Nicaragua, Panama, El Salvador, Venezuela, Peru, Ecuador, Paraguay, Puerto Rico, Chile Bolivia and Uruguay.
Bankok,Thailand-based Global Stem Cells Network (GSCN) to distribute exosome injection and other biological products to stem cell researchers and phsyicians in 15 Latin American countries, including Mexico, Costa Rica, Dominican Republic, Colombia, Argentina, Nicaragua, Panama, El Salvador, Venezuela, Peru, Ecuador, Paraguay, Puerto Rico, Chile Bolivia and Uruguay.
The agreement gives Global Stem Cells Group exclusive distribution rights for the following GSCN products:
- Exosome Injection, a human induced pluripotent stem cell-derived mesenchymal stem cell treatment, which has emerged as a promising supplement to stem cell transplantation therapies. Exosomes derived from mesenchymal stem cells can play an important role in repairing injured tissues. Exosome injection is also utilized as a complement to bone marrow stem cell extractions.
- GcMaf ( Goleic) Injections, a breakthrough technology used to counteract the effects of immune system suppression
 caused by cancerous tumor cells. Complementary oncology utilizes GcMAF therapy to boost the patient’s immune system. Peer reviewed studies have proven that GcMAF Goleic therapy is an immune activator, and can be used as a natural cancer treatment. GcMAF Goleic inhibits blood supply to tumors, activates self-destruction of cancer cells, reverts cancer cells’ characteristics to normal healthy cells, reduces the metastatic potential of human cancer cells and increases energy production at the mitochondrial level.
caused by cancerous tumor cells. Complementary oncology utilizes GcMAF therapy to boost the patient’s immune system. Peer reviewed studies have proven that GcMAF Goleic therapy is an immune activator, and can be used as a natural cancer treatment. GcMAF Goleic inhibits blood supply to tumors, activates self-destruction of cancer cells, reverts cancer cells’ characteristics to normal healthy cells, reduces the metastatic potential of human cancer cells and increases energy production at the mitochondrial level. - Myopep Injection provides a potentially integral part of muscular enhancement and sports medicine rehabilitation, as well as therapeutic care for type 2 diabetes-related weight issues, obesity-related weight loss, aging-related muscle wasting and cerebral palsy. Myopep utilizes bio-engineered peptides to effectively inhibit myostatin, the growth and differentiation factor found in muscle receptor sites. Successful clinical trials demonstrate significant fat loss and muscle gain. Myopep is capable of inducing a pharmaceutical grade effect in human muscle and adipose fat function. Metabolism or glycolysis (sugar metabolism) rises, forcing fat to be broken down into sugar to feed muscular action and growth, causing increased muscle growth, definition and strength. One subcutaneous injection of Myopep at the abdominal area weekly over a period of two months has shown significant fat loss in clinical trials, and a significant drop in glucose metabolic parameters.
“Global Stem Cells Group strives to remain in the forefront of non-invasive, natural medical therapies that provide options for patients who are not finding relief through traditional treatments, and GSCN’s biological products are valuable in achieving these goals,” says Global Stem Cells Group CEO Benito Novas. “As the exclusive distributor of GSNC products in Latin America, this is an excellent opportunity to offer patients access to the most cutting-edge natural remedies available.”
For more information, visit the Global Stem Cells Group website, email info(at)stemcelltraining(dot)net, or call (305) 560-5337.
About Global Stem Cell Group:
Global Stem Cells Group is the parent company of six wholly owned operating companies dedicated entirely to stem cell research, training, products and solutions. Founded in 2012, the company combines dedicated researchers, physician and patient educators and solution providers with the shared goal of meeting the growing worldwide need for leading edge stem cell treatments and solutions. With a singular focus on this exciting new area of medical research, Global Stem Cells Group and its subsidiaries are uniquely positioned to become global leaders in cellular medicine.
Global Stem Cells Group’s corporate mission is to make the promise of stem cell medicine a reality for patients around the world. With each of GSCG’s six operating companies focused on a separate research-based mission, the result is a global network of state-of-the-art stem cell treatments.
About Global Stem Cells Network (GSCN):
Bangkok, Thailand-based Global Stem Cells Network is made up of internationally-trained physicians and health care professionals dedicated to providing the safest, most effective stem cell treatments available today. GSCN is a recognized and trusted leader in providing stem cell specialist treatment programs since 2010, and related medical care since 2003.
To view this press release live online, click here.
###
- Published in Press Releases
- 1
- 2