Gordie Howe’s Stem Cells Treatments Support a Growing Appeal for These Therapies Among Athletes and Baby Boomers
In October, 2014, legendary hockey player Gordie Howe, then 86, was on death’s door after suffering a debilitating acute hemorrhagic, left thalamus stroke. Upon returning home from the hospital, Howe needed someone to lift him from his bed to a wheelchair and back. He couldn’t remember the names of his four children, Marty, Mark, Cathy, and Murray, and his condition continued to grow worse in subsequent weeks. According to an article in New York Magazine, when Mark took his father to get an epidural to relieve his back pain, the attending physician took one look at Gordie and asked Mark if it might be better to just let his father go. On the rare occasion when Gordie did manage to speak, he would tell his children, “Just take me out back and shoot me.”
The man for whom the term “hat trick” was coined, Howe retired from hockey at age 52 after four decades of professional play, having scored more goals than any other NHL player. But over the past 10 years, his health declined dramatically—heart disease, dementia, and spinal stenosis—despite his family’s and physician’s best efforts to find medical solutions. After his stroke, Keith Olbermann aired a preemptive obituary on ESPN. The family made funeral plans. Murray, his youngest son, wrote a eulogy.
Experimental stem cell treatment
Around Thanksgiving, 2015, Howe’s family learned about an experimental stem cell treatment that could save his life. The plan was to inject up to 100 million neural stem cells into his spinal column in the hopes that the stem cells would migrate to his brain and help his body repair itself. Howe could improve within 24 hours, and receive the treatment anytime—just not in the United States. The procedure wasn’t FDA-approved, and Howe would have to receive the treatment at a clinic in Tijuana.
Howe’s son Murray, a radiologist, looked into the treatment and thought it was promising. The real concern was transporting the immobile Howe to Mexico. Daughter Cathy worried that he might die during the treatment, but Mark responded bluntly: “If he does die, what’s the difference? He’s going to be gone soon no matter what.”
While the family weighed the stem cell treatment idea, Howe was admitted to the hospital with severe dehydration, caused by his unwillingness to swallow. When he returned home, he still had no use of his right side, and the family assumed he would never walk again. The Howe children decided to give the stem cell treatments a try.
Two days later, the Howes flew their Dad to San Diego. In the air, Gordie grew agitated and got the attention of a flight attendant, who spent 10 minutes kneeling by his seat trying to understand something he wanted to tell her. Due to his profound memory loss, Howe didn’t know he had suffered a stroke, why he was on a plane, or where he was going. But he remembered one thing, which he managed to whisper to the fight attendant: “I was a pro hockey player.”
The next morning, Marty and Murray drove with their father across the border to Clínica Santa Clarita, where Gordie bent over a table to expose his lower back so that a needle could be inserted into his spinal canal to inject the stem cells. Howe was given two types of stem cells – neural stem cells and mesenchymal stem cells. The second type, derived from bone marrow, has anti-inflammatory properties and secretes chemicals that promote healing.
The procedure then required Howe to lie prone for eight hours. After the eight hours passed, Gordie told Murray he needed to use the bathroom and that he intended to walk there in order to do so. Since the stroke, Gordie had only managed to walk one time—10 steps, with a walker. Murray reminded his Dad that he couldn’t walk.
Recovery milestone after stem cell treatment
Howe stood up, and with Murray’s support, walked for the first time in more than a month—to the bathroom. This milestone became an oft-repeated story among the Howe family, and Gordie’s revival became an irresistible story for the sports pages. Back home, Gordie returned to something resembling the normal life of an 86-year-old. He pushed the grocery cart, helped with the dishes, and could go fishing so long as one of his sons reminded him that a tug on the line meant he needed to start reeling. The family released a video of Gordie standing stationary, firing a puck, five-hole, past his 8-year-old great-grandson. Keith Olbermann apologized for his premature obituary.
Howe’s children now had to figure out how to share his apparent recovery—a debate that proved just as contentious as their decision to fly him to Mexico for the treatment in the first place—with the world. Both Marty and Mark had played in the NHL alongside their father, but now Murray, the doctor, was giving interviews in his hospital scrubs, endorsing his father’s place in medical “miracle” history. He began referring to the stem-cell treatment as the “Gordie Howe Protocol,” and said that his Toledo-based hospital was looking into conducting an FDA-approved study of the procedure. In one interview, Murray Howe stated that “stem cells are the most promising thing in medicine since the discovery of antibiotics.”
As the story spread, the medical community started to question just how miraculous Howe’s recovery had been.
“Companies selling these products are preying on desperate and vulnerable people and exploiting their hope, much like snake-oil salesmen have done throughout most of human history,” wrote Judy Illes and Fabio Rossi, stem-cell experts at the University of British Columbia, in the Vancouver Sun. Even advocates pointed out that, though the field holds great promise, no reputable studies have shown that such a procedure should work.
And yet, for the children of ailing parents, such skepticism doesn’t matter. Murray’s response to one skeptic was, “What would you do for your father?”
Gordie Howe’s therapy would cost an average patient about $30,000.
Athletes, whether playing or retired, have a special need for the regenerative capacity that stem cells are believed to provide. Athletes break bones, strain ligaments, bash knees and wear out cartilage. If stem cells’ restorative capability is proven, they could be considered the latest form of sports medicine.
Since Howe’s treatment in late 2014, two other athletic legends have received stem cell treatments—former quarterbacks Bart Starr and John Brodie. And the rest of the population, particularly aging baby boomers, isn’t far behind.
 Still, while acceptance of stem cell therapy has grown, so have controversies surrounding its use. While clinical trials authorized by the U.S. Food and Drug Administration are rapidly expanding in the U.S., so are treatments outside the regulated system. Patients are going to stem cell clinics in other countries that approve stem cell therapies.
Still, while acceptance of stem cell therapy has grown, so have controversies surrounding its use. While clinical trials authorized by the U.S. Food and Drug Administration are rapidly expanding in the U.S., so are treatments outside the regulated system. Patients are going to stem cell clinics in other countries that approve stem cell therapies.
For its part, the FDA is drafting guidelines, although the U.S. and Canada still trail other countries in approving stem cell treatments.
Last year, the FDA issued draft guidelines to clarify what types of human cell therapy it regulates. The short answer: Most of them, with “limited exceptions,” according to an FDA email sent in response to questions from The San Diego Union-Tribune. These exceptions include cells or tissues that are “minimally manipulated,” not given with any other product and perform the same function in the donor as in the recipient.
All other stem cell therapies are seen as involving human cells, tissues and cellular and tissue-based products – also known as HCT/Ps – regulated by the FDA’s Center for Biologics Evaluation and Research.
“We understand that determining the appropriate regulatory path for HCT/Ps can be challenging, and the FDA is working diligently to develop guidance to help sponsors and physicians understand how to apply federal regulations to this complex and emerging field,” the agency said.
In January 2015, University of California, Davis stem cell researcher and blogger Paul Knoepfler estimated that more than 100 unauthorized stem cell clinics were operating in the United States. Later that year, he increased that estimate to up to 200.
Then on May 6, he wrote in his blog: “We are seeing a flood of professional athletes getting stem cell treatments in the past few years.”
Athletes and others who want these treatments bristle at what they call cumbersome, time-consuming regulations in the U.S. The situation can be urgent for seriously ill patients.
While it hasn’t been proven that the stem cells enabled his recovery, by all indications Gordie Howe’s health has improved significantly since receiving stem cell treatments. In November, 2015, Murray Howe said that his father’s physicians in the U.S. recommended hospice care in the weeks after the stroke, and the family was told he wouldn’t last more than two or three weeks,
“Then, suddenly, he is raking and sweeping and goofing around in the back yard,” Murray said.
###
###
Sources: The San Diego Union-Tribune, New York Magazine
- Published in Corporate News / Blog
Stem Cell Researchers Discover Stem Cells That Might Repair Skull, Face Bones
Scientists may be one step closer to a breakthrough that uses stem cells to replace damaged skull and facial bones in patients who experience a head trauma or undergo cancer surgery requiring repair and reconstructive surgery.
Researchers have discovered and isolated stem cells capable of repairing these bones in mice. The research, led by Takamitsu Maruyama and the research team at the University of Rochester Medical Center in Rochester, N.Y., could also help patients born with a skull deformity known as craniosynostosis, which can lead to developmental delays and pressure on the brain.
 In the study, scientists investigated the role of the Axin2 gene in bone formation and regeneration. They also examined a specific mutation that causes craniosynostosis in mice. Their finding show that stem cells involved in skull formation are contained within this cell population. These cells are specificto the bones in the head and are very different from other stem cells involved in the formation of the bones in the legs and other parts of the body.
In the study, scientists investigated the role of the Axin2 gene in bone formation and regeneration. They also examined a specific mutation that causes craniosynostosis in mice. Their finding show that stem cells involved in skull formation are contained within this cell population. These cells are specificto the bones in the head and are very different from other stem cells involved in the formation of the bones in the legs and other parts of the body.
Tests to uncover these cells could also help physicians detect bone diseases caused by stem cell abnormalities, according to the researchers.
The research was published Feb. 1 in the journal Nature Communications.
- Published in Corporate News / Blog
German Stem Cell Scientists Develop 3-D “Mini-retinas” –New Hope for Restoring Sight in Patients with Retinal Degeneration Caused by Diabetes and Inherited Disorders.
Medical breakthroughs using stem cells are aimed at all parts of the body bones, kidneys, joints, spines–and now, sight.
A German study in March in Stem Cell Reports, reports that scientists have created an efficient way of developing 3D retina organoids leverage the self-organizing properties of stem cells to create diverse multi-cellular tissue proxies.
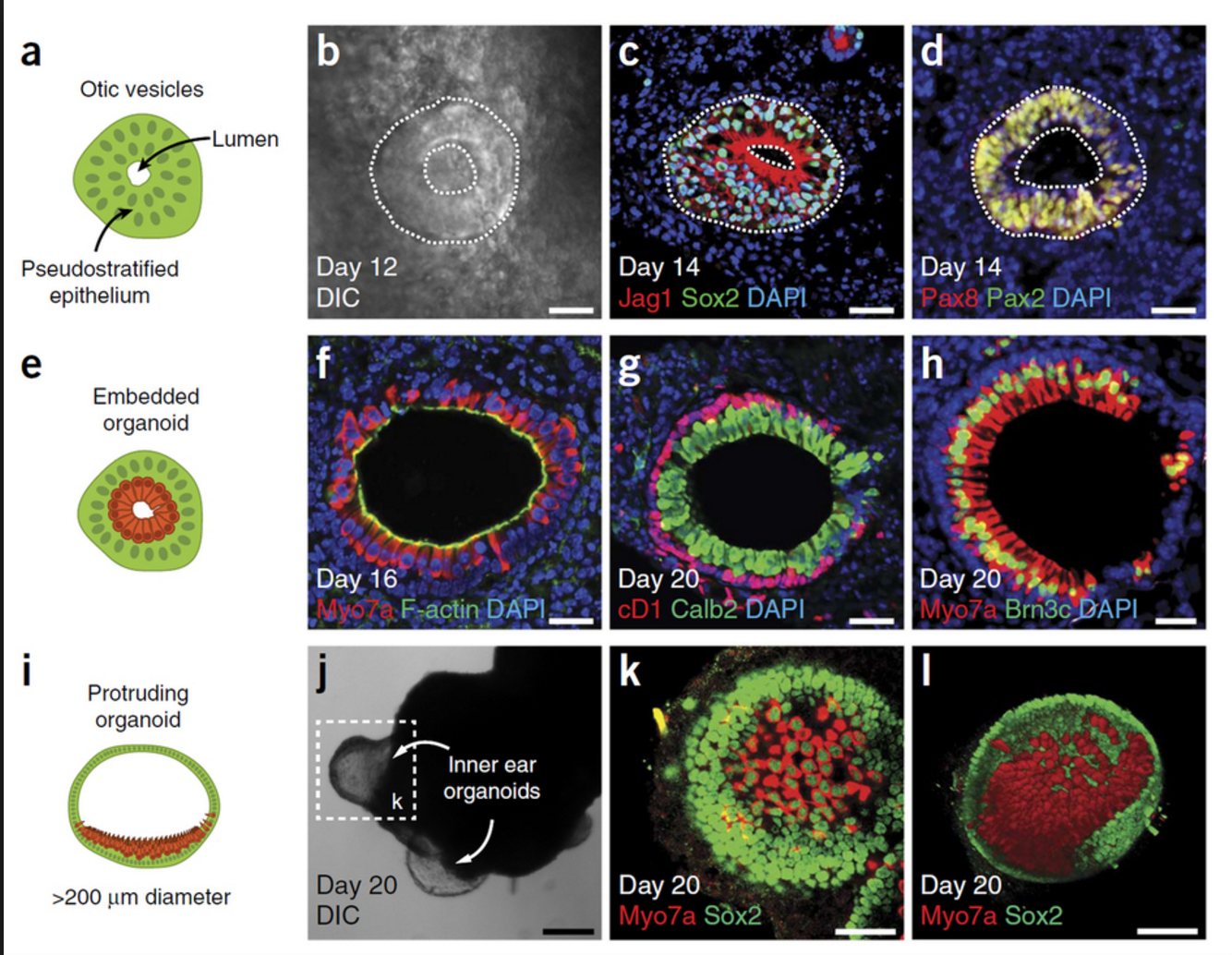
3-D Mini-retinas protocol
The new mini-retina protocol involves cutting an organoid grown from stem cells into three, half-moon shaped pieces at an early stage of eye development. Each of these pieces eventually grows into the full suite of cells found in the retina.
3-D retinal organoids developed in this process efficiently replicate retina formation. This includes the light-detecting cone cells, which now can be produced in high quantities.
cone cells, which now can be produced in high quantities.
Cone photoreceptors, which are responsible for high acuity and color vision, are the most precious retinal cell type with regard to potential future cell replacement therapies in patients affected by retinal degeneration caused by diabetes and inherited disorders.
The process of developing 3D retinal organoids also allows the surviving organoids to grow to reach sizes similar to uncut organoids. These mini-retinas swim around in the dish and because they’re not attached to a surface, better reflect the structure of retinal tissue during development.
In the past, the inability to produce such cells has been a major limitation for regenerative medicine; however, this new method increases the yield of retinal organoids 4-fold, allowing researchers to take a great step forward in the study of the retina and how to repair it.
3-D Mini-retinas offer more diverse ways to study retina tissue
 “The goal isn’t just to make the closest thing next to a real retina, but also to possibly harness the flexibility of the system to create more diverse ways of studying retina tissue,” says senior author Mike Karl, of the German Center for Neurodegenerative Diseases (DZNE) and part of the Center for Regenerative Therapies (CRTD) at Technische Universität Dresden.
“The goal isn’t just to make the closest thing next to a real retina, but also to possibly harness the flexibility of the system to create more diverse ways of studying retina tissue,” says senior author Mike Karl, of the German Center for Neurodegenerative Diseases (DZNE) and part of the Center for Regenerative Therapies (CRTD) at Technische Universität Dresden.
“Even with our new additions to existing organoid systems, we have not yet reached that tipping point of robustness that we need for people without the expertise to grow these models.”
Karl and his colleagues’ comparative studies on pluripotent stem cell-derived human and mouse retina organoids and mouse retina in vivo support the power of the new organoid protocol.
New insights in the study of retinal disease
“Tissue heterogeneity (diversity) is a major challenge in organoid systems, and here our work provides new insight, which will help to develop specific organoid-based models, specifically to reliably study retinal disease mechanism,” says Karl.
The Karl Lab’s change to the mini-retina protocol involves cutting a retina organoid grown from stem cells into three pieces at an early stage of eye development. Each of these pieces, which look like little half moons, eventually grows into the full suite of cells found in the retina, thereby increasing the yield of retinal organoids up to 4-fold compared to previous protocols. Karl’s next objective is to make his 3-D “mini-retinas” even more complex, perhaps by bringing in blood vessels and using the organoids to study regeneration and the function of different neural cell types–specifically, from the human retina.
- Published in Corporate News / Blog
Stem Cell Treatments Normally Used for Cancer Patients are Helping Multiple Sclerosis Patients
The British Broadcasting Corporation (BBC) recently reported that stem cell transplant treatments normally used for cancer patients are helping Multiple Sclerosis (MS) patients in the UK. According to the January 18, 2016 report, 20 patients received bone marrow stem cell transplants using their own stem cells, and that at least some of the patients who were paralyzed by MS are able to walk again post-treatment.
Approximately100,000 people in the United Kingdom suffer from MS, with most new patients diagnosed between the ages of 20 and 30 years of age.
“To have a treatment which can potentially reverse disability is really a major achievement,” says Prof Basil Sharrack, of Sheffield’s Royal Hallamshire Hospital in Sheffield, England.
The treatment, known as autologous hematopoietic stem cell transplantation (HSCT), involves the intravenous infusion of autologous or allogeneic stem cells harvested from the patient’s own bone marrow to reestablish hematopoietic function (formation of blood or blood cells) in patients whose bone marrow or immune system is damaged or defective by chemotherapy. Using stem cells harvested from the patient’s bone marrow helps rebuild the immune system. The theory is that these newly harvested cells are at such an early stage in development that the cellular defects that result in MS do not exist.
“The immune system is being reset or rebooted back to a time point before it caused MS,” says Prof John Snowden, consultant hematologist at Royal Hallamshire Hospital.
The BBC’s Panorama program spoke to several MS patients who have undergone the stem cell transplant.
Steven Storey was diagnosed with MS in 2013 and, within a year, went from being an able-bodied athlete to wheelchair dependent and losing sensation in much of his body.
“I went from running marathons to needing 24-hour acute care. At one point I couldn’t even hold a spoon and feed myself,” Storey says.
Within a few days of the transplant Storey was able to move his toes, and after four months he could stand unaided.
While Storey still needs a wheelchair for mobility, he calls his progress astounding.
“It’s been incredible,” he says. “I was in a dire place, but now I can swim and cycle and I am determined to walk.”
The Royal Hallamshire Hospital along with hospitals in the United States, Sweden and Brazil, is part of an international clinical trial called MIST that is assessing the long-term benefits of the stem cell procedure on MS patients. Study participants all have relapsing remitting MS (RRMS), and received intensive chemotherapy to completely destroy the patients’ immune systems.
Treatment costs are about the same as the annual cost for existing treatments, and the stem cell treatment does not require the use of new or existing medications.
Prof Richard Burt of Northwestern University in Chicago carried out the first hematopoietic stem cell transplantation for MS in 1995, and is coordinating this current MIST international trial, which began in 2006.
“There has been resistance to this in the pharma and academic world,” Burt says. “This is not a technology you can patent and we have achieved this without industry backing.”
A study published last year involving MS patients in Chicago showed significant reductions in neurological disability, and for some the improvements persisted for at least four years, although there was no comparative control group.
The outcomes of the current international trial will be reported in 2018, and may determine whether the stem cell transplant becomes a standard in the United Kingdoms health care system for many MS patients.
“Ongoing research suggests stem cell treatments such as HSCT could offer hope, and it’s clear that in the cases highlighted by Panorama they’ve had a life-changing impact,” says Emma Gray, M.D., head of clinical trials at UK’s MS Society.
- Published in Corporate News / Blog
Our Friend MSCs (Mesenchymal Stem Cells)—Bringing New Life to Old Bones
Researchers from the University of Toronto and The Ottawa Hospital were looking to see if mesenchymal stem cells (MSCs) might treat osteoporosis. MSCs are multipotent stromal cells that can differentiate into a variety of cell types, including: bone cells (osteoblasts), cartilage cells (chondrocytes), muscle cells (myocytes) and fat cells (adipocytes).
Faulty MSCs are the culprits behind osteoporosis; after injecting healthy MSCs into mice with the affliction that causes bones to become weak and brittle, researchers were hoping for a general increase in the mice’s bone health. Instead, they were surprised (and probably very excited) to discover after six months—a quarter of a mouse’s life span—that healthy, functioning bone had replaced the damaged osteoporotic bone. The bone structure in the little creatures, which had been severely compromised by osteoporosis, had been restored to a normal, healthy state! The healthy mesenchymal stem cells did what they were born to do. They became bone cells and went to work, much like the restoration of an old building at the hands of architects and laborers, only without the scaffolds and noise. MSCs work very quietly.
Researchers are hoping that these findings could lead to a new way of treating osteoporosis in humans, or even delay its onset indefinitely.
Stem cell researchers have known for some time that MSCs can boost the regeneration of bone, and in fact a test group of elderly patients in the U.S. who suffer from osteoporosis have already received MSC injections as part of an ancillary trial. The research team is preparing to to examine their blood samples to see if biological markers indicate an improvement in bone growth and bone reabsorption.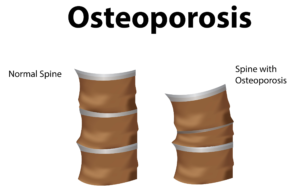
Depending on the outcome of those blood tests, larger trials involving human patients could follow within the next 5 years.
In addition to working quietly and therefore not waking you to the sound of a jackhammer at 7 a.m., there are other cool qualities to MSCs. For instance, they are “a heterogeneous population of musculoskeletal progenitors (another name for adult stem cells) that includes skeletal stem cells (SSCs).” An added perk is that they can be transplanted between individuals without the need to be matched, and without the risk of rejection.
MSCs are awesome.
Globally, more than 200 million people are living with either postmenopausal osteoporosis—known as type 1 osteoporosis, which affects mainly women, or age-related type 2 osteoporosis, which affects both men and women.
With type 2 osteo porosis, there is a reduction in the inner structure of the bone. The bone becomes thinner and less dense, and it can no longer function properly.
porosis, there is a reduction in the inner structure of the bone. The bone becomes thinner and less dense, and it can no longer function properly.
Worldwide, type 2 osteoporosis leads to around 8.9 million bone fractures annually. Hip fractures are among the most common fractures related to osteoporosis, which can lead to disability and even death in elderly patients.
Currently, Teriparatide (brand name Fortéo) is the only drug available to treat type 2 osteoporosis, and its effectiveness lasts for only two years.
The senior author of the study, titled Systemic Mesenchymal Stromal Cell Transplantation Prevents Functional Bone Loss in a Mouse Model of Age-Related Osteoporosis, and published March 17, 2016, is William Stanford, Ph.D., a senior scientist at The Ottawa Hospital Research Institute and a professor at the University of Ottawa. Previous research led Stanford to discover the association between defects in MSC and age-related osteoporosis in mice.
The study’s co-author, John E. Davies, Ph.D., D.Sc., is a professor at the University of Toronto’s Institute of Biomaterials and Biomedical Engineering, The study’s findings are published in the current issue of Stem Cells Translational Medicine.
- Published in Corporate News / Blog
How Stem Cell Therapies Can Help Heal Sports Injuries
Continuing our recent discussion of stem cell therapies for sports injuries, the use of mesanchysmal stem cells (MSCs) in orthopedic medicine can help in the repair of damaged tissue by harnessing the healing power of undifferentiated cells that form all other cells in our bodies. The process involves isolating these stem cells from a sample of your blood, bone marrow or adipose tissue (fat cells), and injecting it into the damaged body part to promote healing. Platelet-rich-plasma (PRP), a concentrated suspension of platelets (blood cells that cause clotting of blood) and growth factors, is also used to assist the process of repair.
Below are some examples of injuries and areas of research involving the use of mesenchymal stem cells (MSCs), which are (adult) tissue stem cells that are not only able to produce copies of themselves, but also able to divide and form bone, cartilage, muscle, and adipose (fat) stem cells when cultured under certain conditions:
Cartilage Damage
Cartilage has long bee n considered as an ideal candidate for cell therapy as it is a relatively simple tissue, composed of one cell type, chondrocytes, and does not have a substantial blood-supply network. Of particular interest to researchers is repair of cartilage tissue in the knee, also called the meniscus of the knee. The meniscus is responsible for distributing the body’s weight at the knee joint when there is movement between the upper and lower leg. Only one third of meniscus cartilage has a blood supply, and as the blood supply allows healing factors and stem cells attached to the blood vessels (called perivascular stem cells) to access the damaged site, the meniscus’s natural lack of blood supply impairs healing of this tissue. Damage to this tissue is common in athletes, and is the target for surgery in 60 percent of patients undergoing knee operations, which usually involves the partial or complete removal of the meniscus, which can lead to long-term cartilage degeneration and osteoarthritis.
n considered as an ideal candidate for cell therapy as it is a relatively simple tissue, composed of one cell type, chondrocytes, and does not have a substantial blood-supply network. Of particular interest to researchers is repair of cartilage tissue in the knee, also called the meniscus of the knee. The meniscus is responsible for distributing the body’s weight at the knee joint when there is movement between the upper and lower leg. Only one third of meniscus cartilage has a blood supply, and as the blood supply allows healing factors and stem cells attached to the blood vessels (called perivascular stem cells) to access the damaged site, the meniscus’s natural lack of blood supply impairs healing of this tissue. Damage to this tissue is common in athletes, and is the target for surgery in 60 percent of patients undergoing knee operations, which usually involves the partial or complete removal of the meniscus, which can lead to long-term cartilage degeneration and osteoarthritis.
Recently, researchers increased their focus on the use of MSCs for treatment of cartilage damage in the knee. Some data from animal models suggest that damaged cartilage undergoes healing more efficiently when MSCs are injected into the injury, and this can be further enhanced if the MSCs are modified to produce growth factors associated with cartilage. It has been shown that once the MSCs are injected into the knee they attach themselves to the site of damage and begin to change into chondrocytes, promoting healing and repair. A small number of completed clinical trials in humans using MSCs to treat cartilage damage have reported some encouraging results, but these studies used very few patients, making it difficult to accurately interpret the results. There are currently a number of ongoing trials using larger groups of patients, and the hope is that these will provide more definite information about the role MSCs play in cartilage repair.
Tendinopathy
Tendinopathy relates to injuries that affect tendons – the long fibrous tissues that connect and transmit force from  muscles to bones. Tendons become strained and damaged through repetitive use, making tendinopathy a common injury among athletes. Tendinopathy has been linked to 30 percent of all running-related injuries, and up to 40 percent of tennis players suffer from some form of elbow tendinopathy or “tennis elbow.” Damage occurs to the collagen fibers that make up the tendon, and this damage is repaired by the body through a process of inflammation and production of new fibers that fuse together with the undamaged tissue. However, this natural healing process can take up to a year to resolve, and results in the formation of a scar on the tendon tissue, reducing the tendon’s natural elasticity, decreasing the amount of energy the tissue can store and resulting in a weakening of tendon.
muscles to bones. Tendons become strained and damaged through repetitive use, making tendinopathy a common injury among athletes. Tendinopathy has been linked to 30 percent of all running-related injuries, and up to 40 percent of tennis players suffer from some form of elbow tendinopathy or “tennis elbow.” Damage occurs to the collagen fibers that make up the tendon, and this damage is repaired by the body through a process of inflammation and production of new fibers that fuse together with the undamaged tissue. However, this natural healing process can take up to a year to resolve, and results in the formation of a scar on the tendon tissue, reducing the tendon’s natural elasticity, decreasing the amount of energy the tissue can store and resulting in a weakening of tendon.
MSCs have the ability to generate cells called tenoblasts that mature into tenocytes. These tenocytes are responsible for producing collagen in tendons. This link between MSCs and collagen is the focus for researchers investigating how stem cells may help treat tendinopathy. Substantial research has been carried out on racehorses as they suffer from high rates of tendinopathy, and the injury is similar to that found in humans. Researchers discovered that by injecting MSCs isolated from an injured horse’s own bone marrow into the damaged tendon, recurrence rates were almost cut in half compared to horses that receive traditional medical management for this type of injury. A later study by the same group showed the MSCs improved repair, resulting in reduced stiffness of the tissue, decreased scarring and better fusion of the new fibers with the existing, undamaged tendon. It is not yet clear if these results are due to MSCs producing new tenocytes or their ability to modulate the environment around the tendinopathy, as described above. These promising results paved the way for the first pilot study in humans.
Bone Repair
 Bones are unique in that they have the ability to regenerate throughout life. Upon injury, such as a fracture, a series of events occur to initiate healing of the damaged bone. Initially there is inflammation at the site of injury, and a large number of signals are sent out. These signals attract MSCs, which begin to divide and increase their numbers. The MSCs then change into either chondrocytes, the cells responsible for making a type of cartilage scaffold, or osteoblasts, the cells that deposit the proteins and minerals that comprise the hard bone on to the cartilage. Finally these new structures are altered to restore shape and function to the repaired bone. A number of studies carried out in animals have demonstrated that direct injection or infusing the blood with MSCs can help heal fractures that previously failed to heal naturally. However, as was the case with tendinopathy, it is not yet clear if these external MSCs work by generating more bone-producing cells or through their ability to reduce inflammation and encourage restoration of the blood supply to injured bone, or both.
Bones are unique in that they have the ability to regenerate throughout life. Upon injury, such as a fracture, a series of events occur to initiate healing of the damaged bone. Initially there is inflammation at the site of injury, and a large number of signals are sent out. These signals attract MSCs, which begin to divide and increase their numbers. The MSCs then change into either chondrocytes, the cells responsible for making a type of cartilage scaffold, or osteoblasts, the cells that deposit the proteins and minerals that comprise the hard bone on to the cartilage. Finally these new structures are altered to restore shape and function to the repaired bone. A number of studies carried out in animals have demonstrated that direct injection or infusing the blood with MSCs can help heal fractures that previously failed to heal naturally. However, as was the case with tendinopathy, it is not yet clear if these external MSCs work by generating more bone-producing cells or through their ability to reduce inflammation and encourage restoration of the blood supply to injured bone, or both.
Brain injury in sports
There is mounting evidence that those taking part in sports where they are exposed to repetitive trauma to the head and brain are at a higher risk of developing neurodegenerative disorders, some of which are targets for stem cell treatments. For example, it has been reported that the rate of these diseases, like Alzheimer Disease, were almost four times higher in professional American football players compared to the general population. While the cause of this disease is not yet clear, it is associated with abnormal accumulation of proteins in neural cells that eventually un dergo cell death and patients develop dementia. Researchers have attempted a number of strategies to investigate treatments of this disease in mice, including introducing neural stem cells that could produce healthy neurons. While some of these experiments have demonstrated positive, if limited, effects, to date there are no stem cell treatments available for Alzheimer’s Disease.
dergo cell death and patients develop dementia. Researchers have attempted a number of strategies to investigate treatments of this disease in mice, including introducing neural stem cells that could produce healthy neurons. While some of these experiments have demonstrated positive, if limited, effects, to date there are no stem cell treatments available for Alzheimer’s Disease.
Boxers suffering from dementia pugilistica, a disease thought to result from damage to nerve cells, can also demonstrate some symptoms of Parkinson’s Disease (among others). In healthy brains, specialized nerve cells called dopaminergic neurons produce dopamine, a chemical that transmits signals to the part of the brain responsible for movement. The characteristic tremor and rigidity associated with Parkinson’s Disease is due to the loss of these dopaminergic neurons and the resulting loss of dopamine production. Researchers are able to use stem cells to generate dopaminergic neurons in the lab that are used to study the development and pathology of this disease. While a recent study reported that dopaminergic neurons derived from human embryonic stem cells improved some symptoms of the disease in mice and rats, stem cell based treatments are still in the development phase.
- Published in Corporate News / Blog
Stem cells and Platelet Rich Plasma Therapies Look Promising for Treating a Variety of sports-related injuries
Stem cell therapies for the treatment of various injuries and diseases that afflict athletes and sportspeople have been the focus of researchers for at least a few years, and recent findings are optimistic.
Stem cell scientists worldwide have been actively pursuing stem cell therapies to harness the process by which stem cells repair and replace damaged tissues and cells.
Researchers at the Mayo Clinic in Rochester, Minnesota are also incorporating platelet rich plasma (PRP) harvested from the patient’s own blood to isolate and concentrate platelets (clotting cells) and then inject them back into the injured area to amend inflammation and initiate the healing process. PRP preparations can be individualized to meet the patient’s specific needs.
The Mayo Clinic is also leveraging stem cells’ ability to regenerate tissues to promote healing. For sports injury therapies stem cells are harvested from the patient’s bone marrow, which also contains other potentially therapeutic cells. Stem cells are powerful, naturally occurring cells that have the ability to modify inflammation and promote natural healing. Mayo Clinic researchers obtain bone marrow from the patient’s pelvic bone, concentrate it to remove the unwanted portions, and inject it back into the injured area.
Global Stem Cells’ associate Joseph Purita, M.D., an osteopathic surgeon and pioneer in the use of stem cells and regenerative medicine techniques to treat sports injuries, uses PRP in concert with stem cells. Dr. Purita has been the focus of much attention for his breakthrough stem cell and PRP treatments to treat injured professional athletes, and has been credited for bringing orthopedic stem cell therapies into the worldwide sports injuries spotlight.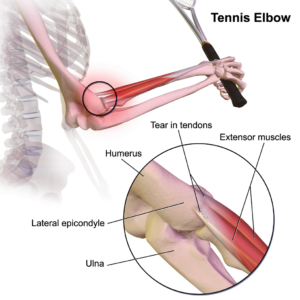
Stem Cell Treatments for Muscle Repair
Forty-five percent of all sports-related injuries involve muscle contusion and strain. Muscle tissue is composed of long, tubular cells called myoblasts—embryonic cells—that fuse to form muscle fibers. Muscle stem cells—also called satellite cells—are responsible for muscle repair. When an individual exercises, muscle fibers become damaged and send signals to these satellite cells, which are perched on top of the muscle tissue. In response to the damaged muscle tissue signals, the satellite cells become activated, begin to divide, replicate themselves and generate new myoblast cells. These myoblasts are then integrated and repair the damaged muscle tissue. Recently, scientists have discovered that satellite cells from older mice are not able to regenerate muscle as efficiently as those from young mice, a discovery that led researchers to identify drugs that restore the function of older cells, which could be used in the future to enhance muscle tissue repair.
Mesenchymal Stem Cells (MSCs) used for injury repair
Mesenchymal stem cells (MSCs) are (adult) tissue stem cells that are not only able to produce copies of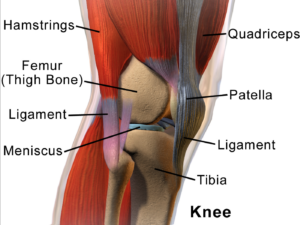 themselves, they can divide and form bone, cartilage, muscle, and adipose (fat) stem cells when cultured under certain conditions. MSCs are attractive to researchers and clinicians since they can be readily isolated from a variety of patient tissues, including fat. Once harvested and, if necessary, elevated to high numbers in culture, MSCs can be re-introduced to the patient from whom they were harvested, eliminating any risk of immune-rejection. In response to injury, MSCs produce proteins that appear to alter the surrounding environment and promote healing and tissue regeneration, such as anti-inflammatory factors, angiogenic factors (which promote the growth of new blood vessels) and other factors that stimulate local, tissue-specific stem cells.
themselves, they can divide and form bone, cartilage, muscle, and adipose (fat) stem cells when cultured under certain conditions. MSCs are attractive to researchers and clinicians since they can be readily isolated from a variety of patient tissues, including fat. Once harvested and, if necessary, elevated to high numbers in culture, MSCs can be re-introduced to the patient from whom they were harvested, eliminating any risk of immune-rejection. In response to injury, MSCs produce proteins that appear to alter the surrounding environment and promote healing and tissue regeneration, such as anti-inflammatory factors, angiogenic factors (which promote the growth of new blood vessels) and other factors that stimulate local, tissue-specific stem cells.
- Published in Corporate News / Blog
Stem Cells are Gaining Momentum Among Smart Investors
Stem cell and regenerative medicine companies are beginning to attract some serious attention from investors who have taken the time to follow advancements in biotechnology, and the explosive movement of these technologies and products into clinical settings worldwide.
There are a lot of dynamics in health care today, and no one should be surprised by the rapid growth in health consumer demand for stem cell therapies. Health care consumers want alternatives to invasive, expensive and not always remedial treatments that conventional medicine and pharmaceuticals offer. Although the US restricted stem cell research in the early 2000s, the research did not die—it emigrated. The rest of the world continued to research and discover the efficacies of embryonic and adult stem cells with amazing results.
Today, demand for stem cell therapies has increased far beyond what the world of Western medicine and pharmaceuticals can provide. While U.S. regulators lag behind the rest of the world in approving cell treatments, more and more patients from the US and Europe are traveling abroad to countries that offer them. Medical tourism is a booming industry, and many regenerative medicine companies are actively marketing directly to patients, facilitating travel for medical procedures—including stem cell treatments. Even while the U.S. government, the medical industry and even some traditional practitioners continue to try wrapping their heads around regenerative medicine and cellular therapies, the field continues to grow. It’s apparent that, no matter what any one government or institution may try to do to control the use of stem cells, the demand for this technology is too intense to stand still.
A constant stream of newly discovered health benefits in this area of biomedicine, combined with health consumer demand has been stimulated and maintained through a flow of increasingly positive studies, clinical trials and media coverage.
It’s only a matter of time before frustration over the disparity between the promise of stem cell treatments and the reality of the very limited access Westerners have to it will burst at the seams.
Today’s health consumers have decided to pursue the many stem cell therapies that are produced outside the conventional medical model, and demand for stem cell therapies has stimulated a global supply of what the Western model’s resistance simply can’t contain forever.
Today’s stem cell companies are entrenched in a segment of the medical market that has the potential to change the way modern medicine is practiced forever.
Committed and enterprising physicians are expanding their practices in record numbers by adding stem cell treatments to their services, and patients demand is dynamic.
Regenerative medicine is attracting some serious capital from investors who understand biotechnology and have the resources to move products into the market, despite the ongoing battles that continue to hover on the political and reimbursement fronts. Benito Novas, CEO of international biotech Global Stem Cells Group, sees near-term milestones ahead, as well as an incursion of validating capital from distinguished investors who believe in the future of stem cell research, gene therapies, and genetically modified cell therapies. The forecast is in favor of the tremendous impact they have the potential to change the model of medicine as we know it.
Patients and practitioners are moving past the existing model of pharmaceutical intervention, treatments and disease management to curative outcomes. In addition to the rapidly growing biotech industry is a growing constituency of patient advocacy organizations and research institutes focused on advanced stem cell therapies and regenerative medicine. This wide ranging constituency increasingly drives how and where stem cell technology can be applied, and exposes the challenges and opportunities encountered by this revolutionary research.
In fact, investment in gene therapies, genetically modified cell therapies, regenerative medicine and stem cells has picked up speed, and data on these investments, not just from the venture capital community, but from the public equity markets and also from large pharmaceutical and biotechnology companies, over the last two years, has risen exponentially. Significant partnerships have been established as well.
Between 2013 and 2014, there are some very notable upswings in stem cell and regenerative medicine investments; venture capital has doubled—$2.1 billion ($2.1B) in 2014, compared to $1B in 2013—while initial public offerings (IPOs) almost tripled, to nearly $1.4B. Private investment in public equity funding rose from $1.4B to $2.53B. Upfront partnership payments rose from $42M in 2013 to $323M in 2014. The growth is unprecedented, and partnerships with big pharmaceutical and biotech companies further validate the expanding maturity of these technologies. Some experts forecast future milestones from deals announced in 2014 could potentially reach $9B, versus $2.4B in potential milestones from deals announced in 2013. The momentum continues to drive new and transformative advances in regenerative therapies.
Ultimately, investors could look back to this period and recognize cell therapy innovators who made a fundamental difference in the practice of medicine in the same way that many of the great scientists of the recombinant DNA generation achieved. Those scientists didn’t just change medicine; they changed the lives of patients.
When you think about the value of cellular therapies to patients and the healthcare system in general, especially with regard to the potential cost savings associated with curative therapies and conventional medicine, the future of healthcare should be a no-brainer.
2016 is expected to be a very important year in terms of clinical translation, clinical data and product approval. Expect a number of important, tangible milestones associated with FDA Phase 2 and Phase 3 data, biologics license application (BLA) submissions, and potentially, product approvals.
Investors are in a position to invest in tomorrow’s medical equivalent of Apple, Microsoft or Google. Biotechnology has been overlooked by investors for too long already, and has the potential to dramatically increase in value, especially in the long-term.
###
- Published in Corporate News / Blog
(Almost) Everything You Wanted to Know About Stem Cells But Were Afraid to Ask
Stem cells have captured the interest of biology nerds, armchair practitioners and everyday individuals for years. Where exactly do they come from and how do they work? When can I have my torn rotary cuff/bum knee /arthritis /(fill in the blank) treated with stem cells? At Global Stem Cells Group, we are making stem cell treatments for a variety of medical conditions available in the physician’s office and out-patient treatment clinics worldwide, and we’re aiming to make them readily available in the U.S. soon, so hang tight.
So, what are stem cells, you ask?
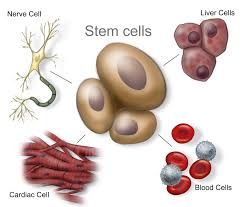 A stem cell is a cell characterized by its ability to self-renew, and its ability to differentiate (change) along multiple pathways. This means a stem cell able is to form specialized cells identical to the cells needed for treatments. For instance, stem cells can become muscle cells, heart cells, skin cells, etc. through stimulation and manipulation via changing chemical environments or genetic triggers. Stem cell physicians know all the tricks to manipulating these uncommitted little units of the body’s rawest materials, and the cells obey!
A stem cell is a cell characterized by its ability to self-renew, and its ability to differentiate (change) along multiple pathways. This means a stem cell able is to form specialized cells identical to the cells needed for treatments. For instance, stem cells can become muscle cells, heart cells, skin cells, etc. through stimulation and manipulation via changing chemical environments or genetic triggers. Stem cell physicians know all the tricks to manipulating these uncommitted little units of the body’s rawest materials, and the cells obey!
So, where to find stem cells when you need them?
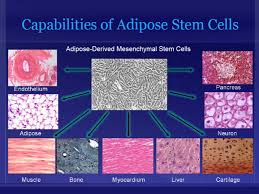 Different types of stem cells come different sources. We like adult adipose-derived stem cells, which are found in abundance in fat—something there seems to be no shortage of! They can be harvested fairly quickly and painlessly from human body fat, which makes them plentiful and readily available. Each liter of fat produces hundreds of millions of potential stem cells. Other stem cells in the body have been examined for their ability to culture usable induced pluripotent stem cells (iPS cells or iPSCs), which are a type of stem cell that can be generated directly from adult cells.
Different types of stem cells come different sources. We like adult adipose-derived stem cells, which are found in abundance in fat—something there seems to be no shortage of! They can be harvested fairly quickly and painlessly from human body fat, which makes them plentiful and readily available. Each liter of fat produces hundreds of millions of potential stem cells. Other stem cells in the body have been examined for their ability to culture usable induced pluripotent stem cells (iPS cells or iPSCs), which are a type of stem cell that can be generated directly from adult cells.
Adult adipose (fat) stem cells appear to be especially prepped for their job, as they are capable of turning into fat, heart, bone or muscle tissue. We know that these fat cells are multipotent, which means they have the ability to self-renew for long periods of time and differentiate into specialized cells with specific functions, thus creating other types of cells.
Today, Global Stem Cells Group has a validated, compliant outpatient method to provide adipose-derived stem cells, as well as bone marrow-derived stem cells, to physicians for use in in-office procedures. We have trained approximately 500 physicians who are currently offering stem cell therapies in their offices and clinics worldwide, and we’ve only scratched the surface.
So, how exactly do stem cells work?
 The concept of stem cell medicine involves the ability to harness the body’s own healing potential to reverse some of the effects of age, degenerative disease and injuries. In other words, we “tell” the stem cells what your body used to be able to do, or how it used to appear, and we have them replicate those youthful, vigorous cells that have not weathered the years or injuries so well.
The concept of stem cell medicine involves the ability to harness the body’s own healing potential to reverse some of the effects of age, degenerative disease and injuries. In other words, we “tell” the stem cells what your body used to be able to do, or how it used to appear, and we have them replicate those youthful, vigorous cells that have not weathered the years or injuries so well.
We often use stem cell therapy interchangeably with regenerative medicine, but this is just one small component of stem cell therapy. We believe the field is going to continue to evolve and move forward, and we’ll be able to combine different techniques to create a truly regenerative response to treatments in patients. The term “stem cell therapy” will eventually become outdated, like when your parents say “groovy.” or “make me a carbon copy.” Instead we will combine techniques like gene therapy and cell therapy in cancers, biologics, scaffolding and delivery systems.
We have really focused on this future in regenerative medicine, combining cellular and gene therapies for use in patients. Everyone in the field is pretty excited about its future, and we believe that regenerative medicine is going to transform medicine as we know it. This is the next big wave for patients who are searching for solutions to their medical needs, but finding few solutions. It’s the 21st century’s version of the polio vaccine, organ transplant and the discovery of antibiotics all rolled up into one—and then some!
- Published in Corporate News / Blog