Our Friend MSCs (Mesenchymal Stem Cells)—Bringing New Life to Old Bones
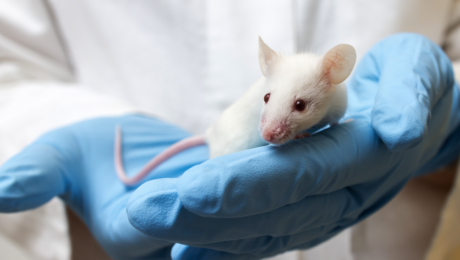
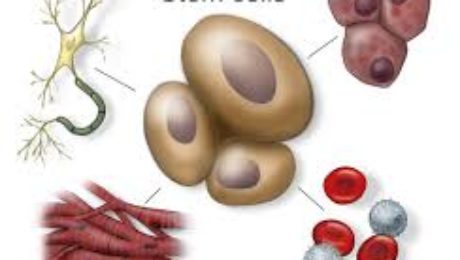
Researchers from the University of Toronto and The Ottawa Hospital were looking to see if mesenchymal stem cells (MSCs) might treat osteoporosis. MSCs are multipotent stromal cells that can differentiate into a variety of cell types, including: bone cells (osteoblasts), cartilage cells (chondrocytes), muscle cells (myocytes) and fat cells (adipocytes).
Faulty MSCs are the culprits behind osteoporosis; after injecting healthy MSCs into mice with the affliction that causes bones to become weak and brittle, researchers were hoping for a general increase in the mice’s bone health. Instead, they were surprised (and probably very excited) to discover after six months—a quarter of a mouse’s life span—that healthy, functioning bone had replaced the damaged osteoporotic bone. The bone structure in the little creatures, which had been severely compromised by osteoporosis, had been restored to a normal, healthy state! The healthy mesenchymal stem cells did what they were born to do. They became bone cells and went to work, much like the restoration of an old building at the hands of architects and laborers, only without the scaffolds and noise. MSCs work very quietly.
Researchers are hoping that these findings could lead to a new way of treating osteoporosis in humans, or even delay its onset indefinitely.
Stem cell researchers have known for some time that MSCs can boost the regeneration of bone, and in fact a test group of elderly patients in the U.S. who suffer from osteoporosis have already received MSC injections as part of an ancillary trial. The research team is preparing to to examine their blood samples to see if biological markers indicate an improvement in bone growth and bone reabsorption.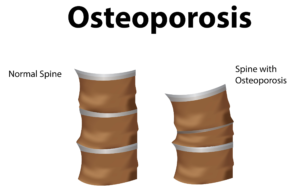
Depending on the outcome of those blood tests, larger trials involving human patients could follow within the next 5 years.
In addition to working quietly and therefore not waking you to the sound of a jackhammer at 7 a.m., there are other cool qualities to MSCs. For instance, they are “a heterogeneous population of musculoskeletal progenitors (another name for adult stem cells) that includes skeletal stem cells (SSCs).” An added perk is that they can be transplanted between individuals without the need to be matched, and without the risk of rejection.
MSCs are awesome.
Globally, more than 200 million people are living with either postmenopausal osteoporosis—known as type 1 osteoporosis, which affects mainly women, or age-related type 2 osteoporosis, which affects both men and women.
With type 2 osteo porosis, there is a reduction in the inner structure of the bone. The bone becomes thinner and less dense, and it can no longer function properly.
porosis, there is a reduction in the inner structure of the bone. The bone becomes thinner and less dense, and it can no longer function properly.
Worldwide, type 2 osteoporosis leads to around 8.9 million bone fractures annually. Hip fractures are among the most common fractures related to osteoporosis, which can lead to disability and even death in elderly patients.
Currently, Teriparatide (brand name Fortéo) is the only drug available to treat type 2 osteoporosis, and its effectiveness lasts for only two years.
The senior author of the study, titled Systemic Mesenchymal Stromal Cell Transplantation Prevents Functional Bone Loss in a Mouse Model of Age-Related Osteoporosis, and published March 17, 2016, is William Stanford, Ph.D., a senior scientist at The Ottawa Hospital Research Institute and a professor at the University of Ottawa. Previous research led Stanford to discover the association between defects in MSC and age-related osteoporosis in mice.
The study’s co-author, John E. Davies, Ph.D., D.Sc., is a professor at the University of Toronto’s Institute of Biomaterials and Biomedical Engineering, The study’s findings are published in the current issue of Stem Cells Translational Medicine.
- Published in Corporate News / Blog
How Stem Cell Therapies Can Help Heal Sports Injuries
Continuing our recent discussion of stem cell therapies for sports injuries, the use of mesanchysmal stem cells (MSCs) in orthopedic medicine can help in the repair of damaged tissue by harnessing the healing power of undifferentiated cells that form all other cells in our bodies. The process involves isolating these stem cells from a sample of your blood, bone marrow or adipose tissue (fat cells), and injecting it into the damaged body part to promote healing. Platelet-rich-plasma (PRP), a concentrated suspension of platelets (blood cells that cause clotting of blood) and growth factors, is also used to assist the process of repair.
Below are some examples of injuries and areas of research involving the use of mesenchymal stem cells (MSCs), which are (adult) tissue stem cells that are not only able to produce copies of themselves, but also able to divide and form bone, cartilage, muscle, and adipose (fat) stem cells when cultured under certain conditions:
Cartilage Damage
Cartilage has long bee n considered as an ideal candidate for cell therapy as it is a relatively simple tissue, composed of one cell type, chondrocytes, and does not have a substantial blood-supply network. Of particular interest to researchers is repair of cartilage tissue in the knee, also called the meniscus of the knee. The meniscus is responsible for distributing the body’s weight at the knee joint when there is movement between the upper and lower leg. Only one third of meniscus cartilage has a blood supply, and as the blood supply allows healing factors and stem cells attached to the blood vessels (called perivascular stem cells) to access the damaged site, the meniscus’s natural lack of blood supply impairs healing of this tissue. Damage to this tissue is common in athletes, and is the target for surgery in 60 percent of patients undergoing knee operations, which usually involves the partial or complete removal of the meniscus, which can lead to long-term cartilage degeneration and osteoarthritis.
n considered as an ideal candidate for cell therapy as it is a relatively simple tissue, composed of one cell type, chondrocytes, and does not have a substantial blood-supply network. Of particular interest to researchers is repair of cartilage tissue in the knee, also called the meniscus of the knee. The meniscus is responsible for distributing the body’s weight at the knee joint when there is movement between the upper and lower leg. Only one third of meniscus cartilage has a blood supply, and as the blood supply allows healing factors and stem cells attached to the blood vessels (called perivascular stem cells) to access the damaged site, the meniscus’s natural lack of blood supply impairs healing of this tissue. Damage to this tissue is common in athletes, and is the target for surgery in 60 percent of patients undergoing knee operations, which usually involves the partial or complete removal of the meniscus, which can lead to long-term cartilage degeneration and osteoarthritis.
Recently, researchers increased their focus on the use of MSCs for treatment of cartilage damage in the knee. Some data from animal models suggest that damaged cartilage undergoes healing more efficiently when MSCs are injected into the injury, and this can be further enhanced if the MSCs are modified to produce growth factors associated with cartilage. It has been shown that once the MSCs are injected into the knee they attach themselves to the site of damage and begin to change into chondrocytes, promoting healing and repair. A small number of completed clinical trials in humans using MSCs to treat cartilage damage have reported some encouraging results, but these studies used very few patients, making it difficult to accurately interpret the results. There are currently a number of ongoing trials using larger groups of patients, and the hope is that these will provide more definite information about the role MSCs play in cartilage repair.
Tendinopathy
Tendinopathy relates to injuries that affect tendons – the long fibrous tissues that connect and transmit force from  muscles to bones. Tendons become strained and damaged through repetitive use, making tendinopathy a common injury among athletes. Tendinopathy has been linked to 30 percent of all running-related injuries, and up to 40 percent of tennis players suffer from some form of elbow tendinopathy or “tennis elbow.” Damage occurs to the collagen fibers that make up the tendon, and this damage is repaired by the body through a process of inflammation and production of new fibers that fuse together with the undamaged tissue. However, this natural healing process can take up to a year to resolve, and results in the formation of a scar on the tendon tissue, reducing the tendon’s natural elasticity, decreasing the amount of energy the tissue can store and resulting in a weakening of tendon.
muscles to bones. Tendons become strained and damaged through repetitive use, making tendinopathy a common injury among athletes. Tendinopathy has been linked to 30 percent of all running-related injuries, and up to 40 percent of tennis players suffer from some form of elbow tendinopathy or “tennis elbow.” Damage occurs to the collagen fibers that make up the tendon, and this damage is repaired by the body through a process of inflammation and production of new fibers that fuse together with the undamaged tissue. However, this natural healing process can take up to a year to resolve, and results in the formation of a scar on the tendon tissue, reducing the tendon’s natural elasticity, decreasing the amount of energy the tissue can store and resulting in a weakening of tendon.
MSCs have the ability to generate cells called tenoblasts that mature into tenocytes. These tenocytes are responsible for producing collagen in tendons. This link between MSCs and collagen is the focus for researchers investigating how stem cells may help treat tendinopathy. Substantial research has been carried out on racehorses as they suffer from high rates of tendinopathy, and the injury is similar to that found in humans. Researchers discovered that by injecting MSCs isolated from an injured horse’s own bone marrow into the damaged tendon, recurrence rates were almost cut in half compared to horses that receive traditional medical management for this type of injury. A later study by the same group showed the MSCs improved repair, resulting in reduced stiffness of the tissue, decreased scarring and better fusion of the new fibers with the existing, undamaged tendon. It is not yet clear if these results are due to MSCs producing new tenocytes or their ability to modulate the environment around the tendinopathy, as described above. These promising results paved the way for the first pilot study in humans.
Bone Repair
 Bones are unique in that they have the ability to regenerate throughout life. Upon injury, such as a fracture, a series of events occur to initiate healing of the damaged bone. Initially there is inflammation at the site of injury, and a large number of signals are sent out. These signals attract MSCs, which begin to divide and increase their numbers. The MSCs then change into either chondrocytes, the cells responsible for making a type of cartilage scaffold, or osteoblasts, the cells that deposit the proteins and minerals that comprise the hard bone on to the cartilage. Finally these new structures are altered to restore shape and function to the repaired bone. A number of studies carried out in animals have demonstrated that direct injection or infusing the blood with MSCs can help heal fractures that previously failed to heal naturally. However, as was the case with tendinopathy, it is not yet clear if these external MSCs work by generating more bone-producing cells or through their ability to reduce inflammation and encourage restoration of the blood supply to injured bone, or both.
Bones are unique in that they have the ability to regenerate throughout life. Upon injury, such as a fracture, a series of events occur to initiate healing of the damaged bone. Initially there is inflammation at the site of injury, and a large number of signals are sent out. These signals attract MSCs, which begin to divide and increase their numbers. The MSCs then change into either chondrocytes, the cells responsible for making a type of cartilage scaffold, or osteoblasts, the cells that deposit the proteins and minerals that comprise the hard bone on to the cartilage. Finally these new structures are altered to restore shape and function to the repaired bone. A number of studies carried out in animals have demonstrated that direct injection or infusing the blood with MSCs can help heal fractures that previously failed to heal naturally. However, as was the case with tendinopathy, it is not yet clear if these external MSCs work by generating more bone-producing cells or through their ability to reduce inflammation and encourage restoration of the blood supply to injured bone, or both.
Brain injury in sports
There is mounting evidence that those taking part in sports where they are exposed to repetitive trauma to the head and brain are at a higher risk of developing neurodegenerative disorders, some of which are targets for stem cell treatments. For example, it has been reported that the rate of these diseases, like Alzheimer Disease, were almost four times higher in professional American football players compared to the general population. While the cause of this disease is not yet clear, it is associated with abnormal accumulation of proteins in neural cells that eventually un dergo cell death and patients develop dementia. Researchers have attempted a number of strategies to investigate treatments of this disease in mice, including introducing neural stem cells that could produce healthy neurons. While some of these experiments have demonstrated positive, if limited, effects, to date there are no stem cell treatments available for Alzheimer’s Disease.
dergo cell death and patients develop dementia. Researchers have attempted a number of strategies to investigate treatments of this disease in mice, including introducing neural stem cells that could produce healthy neurons. While some of these experiments have demonstrated positive, if limited, effects, to date there are no stem cell treatments available for Alzheimer’s Disease.
Boxers suffering from dementia pugilistica, a disease thought to result from damage to nerve cells, can also demonstrate some symptoms of Parkinson’s Disease (among others). In healthy brains, specialized nerve cells called dopaminergic neurons produce dopamine, a chemical that transmits signals to the part of the brain responsible for movement. The characteristic tremor and rigidity associated with Parkinson’s Disease is due to the loss of these dopaminergic neurons and the resulting loss of dopamine production. Researchers are able to use stem cells to generate dopaminergic neurons in the lab that are used to study the development and pathology of this disease. While a recent study reported that dopaminergic neurons derived from human embryonic stem cells improved some symptoms of the disease in mice and rats, stem cell based treatments are still in the development phase.
- Published in Corporate News / Blog
Stem cells and Platelet Rich Plasma Therapies Look Promising for Treating a Variety of sports-related injuries
Stem cell therapies for the treatment of various injuries and diseases that afflict athletes and sportspeople have been the focus of researchers for at least a few years, and recent findings are optimistic.
Stem cell scientists worldwide have been actively pursuing stem cell therapies to harness the process by which stem cells repair and replace damaged tissues and cells.
Researchers at the Mayo Clinic in Rochester, Minnesota are also incorporating platelet rich plasma (PRP) harvested from the patient’s own blood to isolate and concentrate platelets (clotting cells) and then inject them back into the injured area to amend inflammation and initiate the healing process. PRP preparations can be individualized to meet the patient’s specific needs.
The Mayo Clinic is also leveraging stem cells’ ability to regenerate tissues to promote healing. For sports injury therapies stem cells are harvested from the patient’s bone marrow, which also contains other potentially therapeutic cells. Stem cells are powerful, naturally occurring cells that have the ability to modify inflammation and promote natural healing. Mayo Clinic researchers obtain bone marrow from the patient’s pelvic bone, concentrate it to remove the unwanted portions, and inject it back into the injured area.
Global Stem Cells’ associate Joseph Purita, M.D., an osteopathic surgeon and pioneer in the use of stem cells and regenerative medicine techniques to treat sports injuries, uses PRP in concert with stem cells. Dr. Purita has been the focus of much attention for his breakthrough stem cell and PRP treatments to treat injured professional athletes, and has been credited for bringing orthopedic stem cell therapies into the worldwide sports injuries spotlight.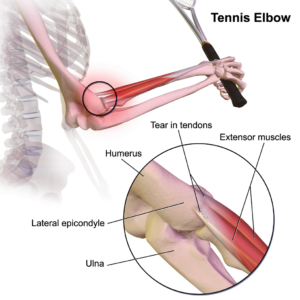
Stem Cell Treatments for Muscle Repair
Forty-five percent of all sports-related injuries involve muscle contusion and strain. Muscle tissue is composed of long, tubular cells called myoblasts—embryonic cells—that fuse to form muscle fibers. Muscle stem cells—also called satellite cells—are responsible for muscle repair. When an individual exercises, muscle fibers become damaged and send signals to these satellite cells, which are perched on top of the muscle tissue. In response to the damaged muscle tissue signals, the satellite cells become activated, begin to divide, replicate themselves and generate new myoblast cells. These myoblasts are then integrated and repair the damaged muscle tissue. Recently, scientists have discovered that satellite cells from older mice are not able to regenerate muscle as efficiently as those from young mice, a discovery that led researchers to identify drugs that restore the function of older cells, which could be used in the future to enhance muscle tissue repair.
Mesenchymal Stem Cells (MSCs) used for injury repair
Mesenchymal stem cells (MSCs) are (adult) tissue stem cells that are not only able to produce copies of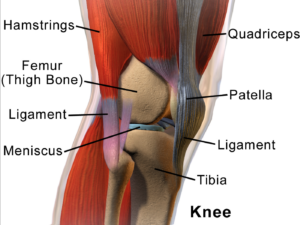 themselves, they can divide and form bone, cartilage, muscle, and adipose (fat) stem cells when cultured under certain conditions. MSCs are attractive to researchers and clinicians since they can be readily isolated from a variety of patient tissues, including fat. Once harvested and, if necessary, elevated to high numbers in culture, MSCs can be re-introduced to the patient from whom they were harvested, eliminating any risk of immune-rejection. In response to injury, MSCs produce proteins that appear to alter the surrounding environment and promote healing and tissue regeneration, such as anti-inflammatory factors, angiogenic factors (which promote the growth of new blood vessels) and other factors that stimulate local, tissue-specific stem cells.
themselves, they can divide and form bone, cartilage, muscle, and adipose (fat) stem cells when cultured under certain conditions. MSCs are attractive to researchers and clinicians since they can be readily isolated from a variety of patient tissues, including fat. Once harvested and, if necessary, elevated to high numbers in culture, MSCs can be re-introduced to the patient from whom they were harvested, eliminating any risk of immune-rejection. In response to injury, MSCs produce proteins that appear to alter the surrounding environment and promote healing and tissue regeneration, such as anti-inflammatory factors, angiogenic factors (which promote the growth of new blood vessels) and other factors that stimulate local, tissue-specific stem cells.
- Published in Corporate News / Blog
Stem Cells are Gaining Momentum Among Smart Investors
Stem cell and regenerative medicine companies are beginning to attract some serious attention from investors who have taken the time to follow advancements in biotechnology, and the explosive movement of these technologies and products into clinical settings worldwide.
There are a lot of dynamics in health care today, and no one should be surprised by the rapid growth in health consumer demand for stem cell therapies. Health care consumers want alternatives to invasive, expensive and not always remedial treatments that conventional medicine and pharmaceuticals offer. Although the US restricted stem cell research in the early 2000s, the research did not die—it emigrated. The rest of the world continued to research and discover the efficacies of embryonic and adult stem cells with amazing results.
Today, demand for stem cell therapies has increased far beyond what the world of Western medicine and pharmaceuticals can provide. While U.S. regulators lag behind the rest of the world in approving cell treatments, more and more patients from the US and Europe are traveling abroad to countries that offer them. Medical tourism is a booming industry, and many regenerative medicine companies are actively marketing directly to patients, facilitating travel for medical procedures—including stem cell treatments. Even while the U.S. government, the medical industry and even some traditional practitioners continue to try wrapping their heads around regenerative medicine and cellular therapies, the field continues to grow. It’s apparent that, no matter what any one government or institution may try to do to control the use of stem cells, the demand for this technology is too intense to stand still.
A constant stream of newly discovered health benefits in this area of biomedicine, combined with health consumer demand has been stimulated and maintained through a flow of increasingly positive studies, clinical trials and media coverage.
It’s only a matter of time before frustration over the disparity between the promise of stem cell treatments and the reality of the very limited access Westerners have to it will burst at the seams.
Today’s health consumers have decided to pursue the many stem cell therapies that are produced outside the conventional medical model, and demand for stem cell therapies has stimulated a global supply of what the Western model’s resistance simply can’t contain forever.
Today’s stem cell companies are entrenched in a segment of the medical market that has the potential to change the way modern medicine is practiced forever.
Committed and enterprising physicians are expanding their practices in record numbers by adding stem cell treatments to their services, and patients demand is dynamic.
Regenerative medicine is attracting some serious capital from investors who understand biotechnology and have the resources to move products into the market, despite the ongoing battles that continue to hover on the political and reimbursement fronts. Benito Novas, CEO of international biotech Global Stem Cells Group, sees near-term milestones ahead, as well as an incursion of validating capital from distinguished investors who believe in the future of stem cell research, gene therapies, and genetically modified cell therapies. The forecast is in favor of the tremendous impact they have the potential to change the model of medicine as we know it.
Patients and practitioners are moving past the existing model of pharmaceutical intervention, treatments and disease management to curative outcomes. In addition to the rapidly growing biotech industry is a growing constituency of patient advocacy organizations and research institutes focused on advanced stem cell therapies and regenerative medicine. This wide ranging constituency increasingly drives how and where stem cell technology can be applied, and exposes the challenges and opportunities encountered by this revolutionary research.
In fact, investment in gene therapies, genetically modified cell therapies, regenerative medicine and stem cells has picked up speed, and data on these investments, not just from the venture capital community, but from the public equity markets and also from large pharmaceutical and biotechnology companies, over the last two years, has risen exponentially. Significant partnerships have been established as well.
Between 2013 and 2014, there are some very notable upswings in stem cell and regenerative medicine investments; venture capital has doubled—$2.1 billion ($2.1B) in 2014, compared to $1B in 2013—while initial public offerings (IPOs) almost tripled, to nearly $1.4B. Private investment in public equity funding rose from $1.4B to $2.53B. Upfront partnership payments rose from $42M in 2013 to $323M in 2014. The growth is unprecedented, and partnerships with big pharmaceutical and biotech companies further validate the expanding maturity of these technologies. Some experts forecast future milestones from deals announced in 2014 could potentially reach $9B, versus $2.4B in potential milestones from deals announced in 2013. The momentum continues to drive new and transformative advances in regenerative therapies.
Ultimately, investors could look back to this period and recognize cell therapy innovators who made a fundamental difference in the practice of medicine in the same way that many of the great scientists of the recombinant DNA generation achieved. Those scientists didn’t just change medicine; they changed the lives of patients.
When you think about the value of cellular therapies to patients and the healthcare system in general, especially with regard to the potential cost savings associated with curative therapies and conventional medicine, the future of healthcare should be a no-brainer.
2016 is expected to be a very important year in terms of clinical translation, clinical data and product approval. Expect a number of important, tangible milestones associated with FDA Phase 2 and Phase 3 data, biologics license application (BLA) submissions, and potentially, product approvals.
Investors are in a position to invest in tomorrow’s medical equivalent of Apple, Microsoft or Google. Biotechnology has been overlooked by investors for too long already, and has the potential to dramatically increase in value, especially in the long-term.
###
- Published in Corporate News / Blog
Demand for Physicians Who Provide Stem Cell Therapies is Growing
There is a growing movement worldwide among patients suffering from degenerative diseases and orthopedic conditions that can’t be treated or cured through conventional medicine for access to stem cell treatments. Fed up with invasive and expensive surgeries, destructive procedures and the side effects of pharmaceuticals, more and more patients and their families are seeking access to the promising world of stem cell therapies.
Global Stem Cells Group is a world leader in stem cell research, training and patient services, and is already providing treatments to patients internationally. But there is a growing call for the science-based medicine community to advocate for appropriate, expeditious FDA action that can make these treatments more available in the U.S.
People suffering from a myriad of conditions who can benefit from stem cell therapies have taken to traveling abroad for them. Global Stem Cells Group has established stem cell treatment clinics worldwide, and conducts research and clinical studies on stem cell therapies routinely.
One of GSCG’s focuses has been on stem cell treatments for COPD, or chronic obstructive pulmonary disease, a progressive condition that makes it difficult to breathe, and gets worse over time. According to the Centers for Disease and Control and Prevention (CDC), more than 1.5 million people in the U.S. have been diagnosed with COPD, which can cause coughing, excess mucus production, wheezing, shortness of breath, chest tightness, and other symptoms that can seriously undermine a person’s quality of life.
We have conducted several studies on the effects of adipose-derived stem cell treatments for COPD, with promising results. In one study using the St. George Respiratory Questionnaire—a disease-specific tool designed to measure impact on overall health, daily life, and perceived well-being in patients with obstructive airways disease—we found that patients demonstrate statistically significant improvements post-treatment, out to the six month time period, including improvements in their ability to complete the six-minute walk distance or exercise capacity.
one study using the St. George Respiratory Questionnaire—a disease-specific tool designed to measure impact on overall health, daily life, and perceived well-being in patients with obstructive airways disease—we found that patients demonstrate statistically significant improvements post-treatment, out to the six month time period, including improvements in their ability to complete the six-minute walk distance or exercise capacity.
Adipose stem cell treatments performed on approximately 40 COPD patients demonstrated marked improvements after therapy, making stem cells a serious contender as a potential healing agent for this debilitating condition. The physician introduces the stem cells through an IV and most of these cells are then caught in the lungs. Adipose stem cells can differentiate to those cells that need to be restored or replaced, including lung tissue cells. In effect, the adipose stem cells remind the lung tissue how to respond when the lungs were healthy, reducing inflammation and promoting healing.
Through our studies, Global Stem Cells Group has seen improvements in patients who suffer from COPD.
We also use adipose stem cells for patients with congestive heart failure. This is a slightly different protocol than the one used for COPD and other conditions. For congestive heart failure patients, instead of injecting differentiated muscle stem cells into the deep scar in the hopes of growing new muscle, we inject the adipose-derived stem cells in the periphery region, where the healthy tissue meets the scar, in the hopes of creating new blood vessels to rejuvenate some of the damaged tissue that hasn’t fully died and turned into scar. By delivering the stem cells via catheter, targeting those areas that have not fully formed a scar, the goal is to increase blood supply to the area. What we have discovered in these studies is that we can increase the injection a fraction—about fifteen percentage points—and we’ve seen statistically significant results demonstrating approximately a 150-meter improvement in the exercise capacity of our congestive heart failure patients.
Additionally, we can combine different cell therapies and different gene therapies or other regenerative medicine techniques to get the best outcome. Moving forward, we will be able to combine adipose-derived stem cells with the muscle-derived stem cells for even better results.
Unfortunately, there are still a lot of regulatory hurdles to overcome in the U.S., but we already offer these therapies at our offshore clinics in Mexico, Panama, the Philippines and other countries. Anyone suffering from a debilitative condition who has hit a roadblock in finding effective treatments in the U.S. should certainly consider learning more about access to stem cell therapies in one of our growing network of international clinics.
 Another very promising trial Global Stem Cells Group completed for critical limb ischemia examined patients who suffered from non-healing lesion wounds on the bottoms of their feet. Most of these patients were diabetic, and as a result had poor perfusion in their limbs. All of the patients were on a list for amputation, so the stem cell trial was a last ditch effort before undergoing limb removal. We began injecting the stem cells intramuscularly along the course of the leg. Thanks to the stem cell treatments, 75 percent of the patients involved were able avoid amputation, with no adverse affects, no deaths, and no patients withdrawn from the study. This was a phenomenal outcome, considering that all of these patients were scheduled for amputation.
Another very promising trial Global Stem Cells Group completed for critical limb ischemia examined patients who suffered from non-healing lesion wounds on the bottoms of their feet. Most of these patients were diabetic, and as a result had poor perfusion in their limbs. All of the patients were on a list for amputation, so the stem cell trial was a last ditch effort before undergoing limb removal. We began injecting the stem cells intramuscularly along the course of the leg. Thanks to the stem cell treatments, 75 percent of the patients involved were able avoid amputation, with no adverse affects, no deaths, and no patients withdrawn from the study. This was a phenomenal outcome, considering that all of these patients were scheduled for amputation.
Stem cell therapies stand to be a powerful approach to treating conditions involving a lack of blood flow in the ischemic tissue, and it doesn’t take too much imagination to see how you can apply this concept to many other conditions and diseases. By utilizing stem cells to help promote angiogenesis (the development of new blood cells), which in turn promotes healing, we expect to revolutionize the way many conditions are treated.
In a first-ever documented case completed at the Hospital Angeles at the Regenerative Medicine Institute, a non-healing leg ulcer resulting from the removal of a cancerous growth had led to only one remedy: amputation of the leg. The patient had received the maximum billable amount of radiation, which destroyed all of the vasculature (blood vessels) in her leg. After undergoing several debridements and an attempted skin graft that proved unsuccessful, amputation was the only available remedy. We gained approval from the Institutional Review Board (IRB) to inject adipose stem cells in and around the wound, although we were unsure of whether it would work since she had normal vasculature leading right up to the point of the wound, where it was basically dead. There was no telling if it could be regenerated.
Eight months post-stem cell therapy, the wound was completely closed and, according to the patient’s radiologist, she had regained normal blood flow in the leg.
For physicians, the ability to provide a wide range of regenerative therapies that can make a substantial quality of life impact on patients who suffer from debilitating orthopedic and degenerative conditions is monumental. As a physician, stem cell certification can help you change lives and create an additional revenue stream for your practice. Regenerative medicine is here to stay. It is already revolutionizing the practice of medicine as we know it, and will continue to gain traction. Patients are actively seeking experienced providers, and Global Stem Cells Group—among the most experienced in this field— offers one of the most comprehensive regenerative medicine programs available.
There’s no more natural or less invasive therapy than using stem cells to heal the body, without the side effects of traditional pharmaceuticals. More conditions are being successfully treated with regenerative medicine therapies today than ever before. As a physician, once you’re experienced in providing some of these regenerative medicine techniques, you can be sure you will have a sustainable, competitive advantage that will benefit your patients and your practice.
- Published in Corporate News / Blog
What’s the Skinny on Adipose Stem Cells Derived From Fat?
In this blog, I’ll share some of the results we’ve had using stem cell therapies in different ways to show you how you can utilize them in your office or clinic. Let’s start with stem cell treatments for cosmetic regenerative tissue enhancement. The procedure starts with taking fat from one location on the patient’s body and relocating it to the area you’re trying to enhance and combining that fat with a population of adipose (fat-derived) stem cells for best results.
This theory, in part, was first published back in 2006 by Kotaro Yoshimura, M.D., Associate Professor, Department of PlasticSurgery at the University of Tokyo. Dr. Yoshimura demonstrated that stem cells harvested from fat are actually responsible for creating new adipocytes.
Does this mean fat is our friend? When it comes to therapeutic tissue treatments, it sure is!
We used to believe that we had a set number of adipocytes and that these either grew or shrank depending on the amount of fat that our bodies were gaining or holding, but we now know better. Everyone has a population of stem cells that exist within their fat tissue that is responsible for replacing or replenishing mature adipocytes, and they’ll grow with weight gain. By attaching to fat tissue, those stem cells will actually help support expansion or weight gain. Therefore, you can take stem cells from one sample of fat—imagine all those stem cells clinging to your fat stores—and put them into another sample of fat to create a cell-enriched population that can be utilized to help create angiogenesis (the development of new blood vessels) and help the graft survive better.
 Take, for instance, breast augmentation using this process. By taking fat from one location, relocating it, and adding stem cells with the fat to the breast tissue, you can reduce reabsorption of the fat tissue. In addition to being able to perform fat transfer for breast augmentation, you can also utilize the stem cells and platelet-rich plasma (PRP) when you need to rejuvenate the skin as well. One example would be a patient who had received an injection of stem cells plus platelet rich plasma without a fat graft: in this case, the cell will be very angiogenic in nature, creating new blood vessels and generating a youthful glow. The cells can also help with collagen production so the patient gets smoother skin and help with scarring or the appearance of unevenness on the skin.
Take, for instance, breast augmentation using this process. By taking fat from one location, relocating it, and adding stem cells with the fat to the breast tissue, you can reduce reabsorption of the fat tissue. In addition to being able to perform fat transfer for breast augmentation, you can also utilize the stem cells and platelet-rich plasma (PRP) when you need to rejuvenate the skin as well. One example would be a patient who had received an injection of stem cells plus platelet rich plasma without a fat graft: in this case, the cell will be very angiogenic in nature, creating new blood vessels and generating a youthful glow. The cells can also help with collagen production so the patient gets smoother skin and help with scarring or the appearance of unevenness on the skin.
Adipose stem cells can also be utilized for regenerative results in orthopedics. A typical technique is to isolate the platelet rich plasma from the peripheral blood and combine it with stem cells from the fat tissue. Our preference is to utilize the adipose stem cells, again, because of the massive volume of stem cells fat tissue delivers. We can obtain about five hundred times more mesenchymal type stem cells—stem cells that that can differentiate into a variety of cell types—from adipose tissue than we can obtain from bone marrow. For this reason, in most cases we utilize the cells from the adipose tissue rather than the bone marrow.
This protocol comes courtesy of Joseph Purita, M.D., a member of the Global Stem Cells Group Advisory Board and a pioneer in the use of stem cells and PRP therapy for orthopedic surgery. Dr. Purita’s protocol is to inject the adipose cells plus the PRP interarticularly to the affected joint.
This therapy has also been used successfully in animals. For instance, in the case of a horse with a chronic, non-healing tear in the ligament considered so chronic that they were going to put it down, an injection of the platelet rich plasma plus the adipose stem cells directly into the lesion resulted in a complete resolution of the non-healing ligament within six months post-treatment.
 Again, courtesy of Dr. Purita, is another example of a patient with avascular necrosis who had been told that she needed a total knee replacement. She was getting her knee drained once per week, had severe swelling and pain, and was not able to perform, pretty much, any activities due to her joint pain. After injection of the adipose cells plus the PRP, the patient was essentially pain free, she was able to play tennis weekly, and there was complete resolution of the avascular necrosis, according to MRIs six months post-treatment.
Again, courtesy of Dr. Purita, is another example of a patient with avascular necrosis who had been told that she needed a total knee replacement. She was getting her knee drained once per week, had severe swelling and pain, and was not able to perform, pretty much, any activities due to her joint pain. After injection of the adipose cells plus the PRP, the patient was essentially pain free, she was able to play tennis weekly, and there was complete resolution of the avascular necrosis, according to MRIs six months post-treatment.
Another example is a patient who was hit by a bus and thrown into a house, resulting in a non-union bone fracture that never healed properly. In this case, the patient was treated at the Hospital Angeles in collaboration with the Regenerative Medicine Institute. The patient had not been able to bear weight on the leg for more than two years. After an injection of stem cells plus a bone matrix, at the three-month follow-up there was full continuity down the length of the bone, and for the first time in more than two years, the patient was able to bear weight.
Treatments using adipose (fat)-derived stem cells, in combination with PRP and other regenerative medicine therapies, are proving to provide the body with the ability to heal in cases where nothing else worked. Initial findings tell us that PRP assisted stem cells can figure out what cells they need to replicate—whether cartilage cells, bone cells, or collagen cells for ligaments and tendons—to help the body heal from within.
- Published in Corporate News / Blog
Supercharge your Regenerative Medicine Education
Global Stem Cells Group is proud to present our 2016 Edition of our Regenerative Medicine Symposium, to be held in 6 different Cities around the World.
This prestigious event will have the presence of a select group of renowned international speakers who will offer a combined day of conferences of high scientific rigor aimed at Physicians.
Global Stem Cells Group’s symposium will provide cutting-edge information on developments in all areas of stem cell research, including the biology, medicine, applications, regulations, product development, and the commercialization of stem cells.
Business opportunities, challenges, and potential strategies for overcoming these challenges will also be addressed. Come join us to learn what categories of companies are currently commercially viable, how they’re being funded, and what kind of strategic relationships are available within the industry
Our First Event of the Year will Take Place in Bogota Colombia in March 3 rd for more info and registration click here http://stemcellconference.org/es/simposio-bogota
- Published in Corporate News / Blog
Essential Steps for Regenerative Medicine Practitioners
Global Stem Cells Group offers a stem cell training course that can help you bring some of our cutting edge regenerative therapies to your practice or clinic. We offer an intensive, hands-on two day training class, show you how to collect fat tissue via our precision mini lipo-aspiration technique, and we walk your through the process of collecting bone marrow via the iliac crest.
We provide didactic lectures on stem cell structure, function and treatment, and we go through the techniques to isolate and harvest stem cells from fat tissue and bone marrow, as well as the platelet rich plasma from the peripheral blood. We do all of this on actual patients! Three to four of them during the two-day course period, so that you can get a good understanding on how to perform the different techniques and procedures.
GSCG will provide you with written protocols, forms and consent so that you can easily implement this in your practice after certification. We also offer instructional videos, quality control (QC) assays, viability and cell counts. Our courses are fully accredited— providing 16 categories for one credit—by three different universities, and we offer the opportunity to participate in Institutional Review Board (IRB) clinical studies.
Now, you’re probably wondering about our products, without which you’d have the weekend off.
For the bone marrow product, we’ve partnered with a company called Emcyte. All of the kits for the bone marrow and the platelet rich plasma are FDA 510K approved, thus allowing for the collection of a small sample of blood, which can be safely attained, to produce concentrated growth factors and platelets. The system is gentle and processes bone marrow aspirate precisely for the purest concentration of cells at the point of care.
Also, there’s our —adipose-derived stem cell Kit . fact: fat tissue is one of the most plentiful sources of stem cells in the body—in particular, the mesenchymal type stem cells. Imagine, you can get about five hundred times more mesenchymal stem cells from fat tissue than you can get from the bone marrow. Fat tissue is a perfect source of stem cells when you’re treating degenerative type diseases, or replenishing some of the tissue that has been damaged as a result of injury and or degenerative disease.
Within this population there exists a multipotential progenitor cell that has the ability to go down the adipogenesis pathway, the chondrogenesis and the osteogenic pathway. These cells are very angiogenic and vasculogenic in nature, meaning they are able to form blood vessels and are very useful in ischemia type conditions.
 Global Stem Cells Group has a variety of isolation kits, and all of our kits are produced according to good manufacturing processes as I mentioned before. We have a full scale laboratory in Santiago Chile where we produce all of the reagents that are necessary, and our kits include all of the consumables necessary to isolate regenerative stem cells. You can visit the Adimarket.net website to view the variety of different products we offer, all of which can assist with some of the work you are doing in-clinic. Including things like centrifuges and other medical devices and equipment that are necessary to bring stem cell therapies to the patients. We also go over this information during the stem cell training course and teach you exactly what equipment you will need to perform some of the techniques.
Global Stem Cells Group has a variety of isolation kits, and all of our kits are produced according to good manufacturing processes as I mentioned before. We have a full scale laboratory in Santiago Chile where we produce all of the reagents that are necessary, and our kits include all of the consumables necessary to isolate regenerative stem cells. You can visit the Adimarket.net website to view the variety of different products we offer, all of which can assist with some of the work you are doing in-clinic. Including things like centrifuges and other medical devices and equipment that are necessary to bring stem cell therapies to the patients. We also go over this information during the stem cell training course and teach you exactly what equipment you will need to perform some of the techniques.
- Published in Corporate News / Blog
(Almost) Everything You Wanted to Know About Stem Cells But Were Afraid to Ask
Stem cells have captured the interest of biology nerds, armchair practitioners and everyday individuals for years. Where exactly do they come from and how do they work? When can I have my torn rotary cuff/bum knee /arthritis /(fill in the blank) treated with stem cells? At Global Stem Cells Group, we are making stem cell treatments for a variety of medical conditions available in the physician’s office and out-patient treatment clinics worldwide, and we’re aiming to make them readily available in the U.S. soon, so hang tight.
So, what are stem cells, you ask?
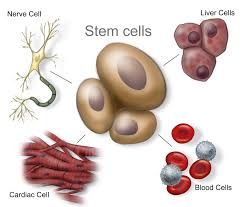 A stem cell is a cell characterized by its ability to self-renew, and its ability to differentiate (change) along multiple pathways. This means a stem cell able is to form specialized cells identical to the cells needed for treatments. For instance, stem cells can become muscle cells, heart cells, skin cells, etc. through stimulation and manipulation via changing chemical environments or genetic triggers. Stem cell physicians know all the tricks to manipulating these uncommitted little units of the body’s rawest materials, and the cells obey!
A stem cell is a cell characterized by its ability to self-renew, and its ability to differentiate (change) along multiple pathways. This means a stem cell able is to form specialized cells identical to the cells needed for treatments. For instance, stem cells can become muscle cells, heart cells, skin cells, etc. through stimulation and manipulation via changing chemical environments or genetic triggers. Stem cell physicians know all the tricks to manipulating these uncommitted little units of the body’s rawest materials, and the cells obey!
So, where to find stem cells when you need them?
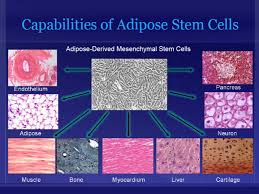 Different types of stem cells come different sources. We like adult adipose-derived stem cells, which are found in abundance in fat—something there seems to be no shortage of! They can be harvested fairly quickly and painlessly from human body fat, which makes them plentiful and readily available. Each liter of fat produces hundreds of millions of potential stem cells. Other stem cells in the body have been examined for their ability to culture usable induced pluripotent stem cells (iPS cells or iPSCs), which are a type of stem cell that can be generated directly from adult cells.
Different types of stem cells come different sources. We like adult adipose-derived stem cells, which are found in abundance in fat—something there seems to be no shortage of! They can be harvested fairly quickly and painlessly from human body fat, which makes them plentiful and readily available. Each liter of fat produces hundreds of millions of potential stem cells. Other stem cells in the body have been examined for their ability to culture usable induced pluripotent stem cells (iPS cells or iPSCs), which are a type of stem cell that can be generated directly from adult cells.
Adult adipose (fat) stem cells appear to be especially prepped for their job, as they are capable of turning into fat, heart, bone or muscle tissue. We know that these fat cells are multipotent, which means they have the ability to self-renew for long periods of time and differentiate into specialized cells with specific functions, thus creating other types of cells.
Today, Global Stem Cells Group has a validated, compliant outpatient method to provide adipose-derived stem cells, as well as bone marrow-derived stem cells, to physicians for use in in-office procedures. We have trained approximately 500 physicians who are currently offering stem cell therapies in their offices and clinics worldwide, and we’ve only scratched the surface.
So, how exactly do stem cells work?
 The concept of stem cell medicine involves the ability to harness the body’s own healing potential to reverse some of the effects of age, degenerative disease and injuries. In other words, we “tell” the stem cells what your body used to be able to do, or how it used to appear, and we have them replicate those youthful, vigorous cells that have not weathered the years or injuries so well.
The concept of stem cell medicine involves the ability to harness the body’s own healing potential to reverse some of the effects of age, degenerative disease and injuries. In other words, we “tell” the stem cells what your body used to be able to do, or how it used to appear, and we have them replicate those youthful, vigorous cells that have not weathered the years or injuries so well.
We often use stem cell therapy interchangeably with regenerative medicine, but this is just one small component of stem cell therapy. We believe the field is going to continue to evolve and move forward, and we’ll be able to combine different techniques to create a truly regenerative response to treatments in patients. The term “stem cell therapy” will eventually become outdated, like when your parents say “groovy.” or “make me a carbon copy.” Instead we will combine techniques like gene therapy and cell therapy in cancers, biologics, scaffolding and delivery systems.
We have really focused on this future in regenerative medicine, combining cellular and gene therapies for use in patients. Everyone in the field is pretty excited about its future, and we believe that regenerative medicine is going to transform medicine as we know it. This is the next big wave for patients who are searching for solutions to their medical needs, but finding few solutions. It’s the 21st century’s version of the polio vaccine, organ transplant and the discovery of antibiotics all rolled up into one—and then some!
- Published in Corporate News / Blog
Could stem cells repair the damaged brain in Alzheimer’s?
Stem cell therapies may hold the cure to Alzheimer’s, although so far that cure has been elusive. People who suffer from Alzheimer’s disease experience disorientation regarding time and place, changes in mood, personality and behavior, memory loss, difficulty solving problems or planning, and difficulty writing or performing other routine and familiar tasks. This progressive and irreversible brain disorder may affect judgment, initiative and social life, and can lead to physical symptoms such as vision problems.
Alzheimer’s affects mostly people aged 70 years and above, and is more common in women. It is the main risk factor for dementia among the elderly.
There is no known cure for Alzheimer’s. Conventional treatments, both drug-based and non-drug strategies, may help with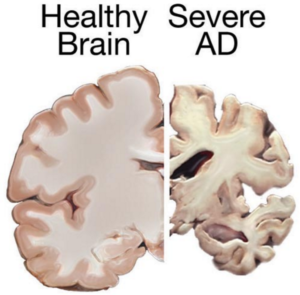 cognitive and behavioral symptoms, but have little to no effect on the disease’s development over the long term. Current medications can’t stop Alzheimer’s from progressing, but they can temporary lessen symptoms like confusion and memory loss.
cognitive and behavioral symptoms, but have little to no effect on the disease’s development over the long term. Current medications can’t stop Alzheimer’s from progressing, but they can temporary lessen symptoms like confusion and memory loss.
Although there have been attempts to find a remedy for Alzheimer’s, and despite the fact that scientists have managed to effectively treat lab animals with drug-based treatments, no animal model has managed to truly mimic its symptoms as they manifest in humans. Remedies that worked in lab animals have failed to work in humans; for this reason, scientists decided to try a different approach by exploring the possibilities of stem cells therapies in Alzheimer’s treatments.
Can Stem cells develop new Alzheimer’s treatments?
Alzheimer’s disease affects neurons in all parts of the brain, and the complexity of this condition makes it difficult to create a model that perfectly mimics its manifestations. At least in theory, stem cells could be used for treating this condition by transplanting neural stem cells into the patient’s brain in an attempt to generate healthy new neurons to replace dead and damaged neurons. It remains unclear whether the brain would be able to integrate the transplanted cells, and if the neural stem cells are able to travel to the damaged areas.
Another great challenge is producing the different types of neurons needed to replace the damaged cells, and to find a way to stimulate the renewal of the lost connections between neural cells. Even if the transplanted cells survive and find their way to the damaged areas, they might become damaged by proteins that build up in the brain—the same proteins that cause the disease in the first place, which means any effects of a stem cell transplant could be only temporary.
 A different approach would be to use stem cells for delivering neurotrophins to the brain. Neurotrophin proteins support the growth and survival of neurons, but in patients with Alzheimer’s, they’re produced in amounts too low to contribute such support. Neural stem cells can produce such cells, and in mice tests this method did prove helpful; scientists observed some improvements in memory in mice treated with stem cells.
A different approach would be to use stem cells for delivering neurotrophins to the brain. Neurotrophin proteins support the growth and survival of neurons, but in patients with Alzheimer’s, they’re produced in amounts too low to contribute such support. Neural stem cells can produce such cells, and in mice tests this method did prove helpful; scientists observed some improvements in memory in mice treated with stem cells.
Mesenchymal stem cells could also be used for treating Alzheimer’s—not to replace damaged neurons, but to heal them. Mesenchymal stem cells may exert anti-inflammatory effects and may help ameliorate the symptoms of Alzheimer’s, but there’s no study at the moment to prove their safety or effectiveness in this condition [3].
Although clinical trials and studies on Alzheimer’s disease treatment to date have a high failure rate, the use of stem cells may be helpful for studying the behavior of brain cells damaged by the condition, as well as for testing various therapeutic approaches and predicting which treatment may help Alzheimer’s patients.
Researchers from the Harvard Stem Cells Institute took skin cells from Alzheimer’s patients and reprogramed them to create induced pluripotent stem cells (iPSC); obtained cells were grown in special lab conditions and were found to release the same proteins that form plaque in Alzheimer’s patients [2]. This may give scientists the opportunity to study the behavior of Alzheimer’s-affected cells and to search for and test new remedies.
Asian scientists managed to turn human fibroblasts into neuronal cells using a chemical cocktail of small molecules [6]. These findings may provide an alternative strategy for modeling the neurodegenerative disorder, which may help scientists understand the mechanisms behind this condition. It may also play an important role in identifying new stem cell based treatments.
References
- http://www.eurostemcell.org/factsheet/alzheimer%E2%80%99s-disease-how-could-stem-cells-help
- http://hsci.harvard.edu/news/alzheimer%E2%80%99s-dish
- http://www.ipscell.com/2012/05/can-stem-cells-be-used-to-treat-alzheimers-disease/
- http://www.sciencedirect.com/science/article/pii/S1934590915003173
- http://www.cell.com/cell-stem-cell/abstract/S1934-5909%2815%2900305-7
- Published in Corporate News / Blog