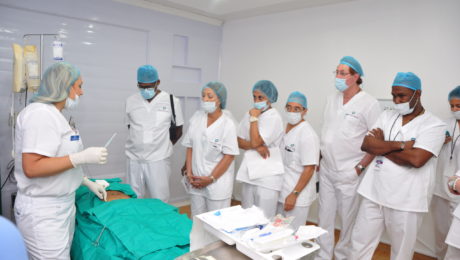
Supercharge your Regenerative Medicine Education
Global Stem Cells Group is proud to present our 2016 Edition of our Regenerative Medicine Symposium, to be held in 6 different Cities around the World.
This prestigious event will have the presence of a select group of renowned international speakers who will offer a combined day of conferences of high scientific rigor aimed at Physicians.
Global Stem Cells Group’s symposium will provide cutting-edge information on developments in all areas of stem cell research, including the biology, medicine, applications, regulations, product development, and the commercialization of stem cells.
Business opportunities, challenges, and potential strategies for overcoming these challenges will also be addressed. Come join us to learn what categories of companies are currently commercially viable, how they’re being funded, and what kind of strategic relationships are available within the industry
Our First Event of the Year will Take Place in Bogota Colombia in March 3 rd for more info and registration click here http://stemcellconference.org/es/simposio-bogota
- Published in Corporate News / Blog
Essential Steps for Regenerative Medicine Practitioners
Global Stem Cells Group offers a stem cell training course that can help you bring some of our cutting edge regenerative therapies to your practice or clinic. We offer an intensive, hands-on two day training class, show you how to collect fat tissue via our precision mini lipo-aspiration technique, and we walk your through the process of collecting bone marrow via the iliac crest.
We provide didactic lectures on stem cell structure, function and treatment, and we go through the techniques to isolate and harvest stem cells from fat tissue and bone marrow, as well as the platelet rich plasma from the peripheral blood. We do all of this on actual patients! Three to four of them during the two-day course period, so that you can get a good understanding on how to perform the different techniques and procedures.
GSCG will provide you with written protocols, forms and consent so that you can easily implement this in your practice after certification. We also offer instructional videos, quality control (QC) assays, viability and cell counts. Our courses are fully accredited— providing 16 categories for one credit—by three different universities, and we offer the opportunity to participate in Institutional Review Board (IRB) clinical studies.
Now, you’re probably wondering about our products, without which you’d have the weekend off.
For the bone marrow product, we’ve partnered with a company called Emcyte. All of the kits for the bone marrow and the platelet rich plasma are FDA 510K approved, thus allowing for the collection of a small sample of blood, which can be safely attained, to produce concentrated growth factors and platelets. The system is gentle and processes bone marrow aspirate precisely for the purest concentration of cells at the point of care.
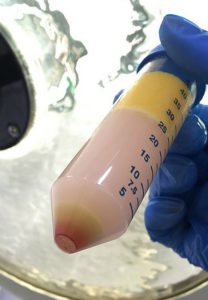
Also, there’s our —adipose-derived stem cell Kit . fact: fat tissue is one of the most plentiful sources of stem cells in the body—in particular, the mesenchymal type stem cells. Imagine, you can get about five hundred times more mesenchymal stem cells from fat tissue than you can get from the bone marrow. Fat tissue is a perfect source of stem cells when you’re treating degenerative type diseases, or replenishing some of the tissue that has been damaged as a result of injury and or degenerative disease.
Within this population there exists a multipotential progenitor cell that has the ability to go down the adipogenesis pathway, the chondrogenesis and the osteogenic pathway. These cells are very angiogenic and vasculogenic in nature, meaning they are able to form blood vessels and are very useful in ischemia type conditions.
 Global Stem Cells Group has a variety of isolation kits, and all of our kits are produced according to good manufacturing processes as I mentioned before. We have a full scale laboratory in Santiago Chile where we produce all of the reagents that are necessary, and our kits include all of the consumables necessary to isolate regenerative stem cells. You can visit the Adimarket.net website to view the variety of different products we offer, all of which can assist with some of the work you are doing in-clinic. Including things like centrifuges and other medical devices and equipment that are necessary to bring stem cell therapies to the patients. We also go over this information during the stem cell training course and teach you exactly what equipment you will need to perform some of the techniques.
Global Stem Cells Group has a variety of isolation kits, and all of our kits are produced according to good manufacturing processes as I mentioned before. We have a full scale laboratory in Santiago Chile where we produce all of the reagents that are necessary, and our kits include all of the consumables necessary to isolate regenerative stem cells. You can visit the Adimarket.net website to view the variety of different products we offer, all of which can assist with some of the work you are doing in-clinic. Including things like centrifuges and other medical devices and equipment that are necessary to bring stem cell therapies to the patients. We also go over this information during the stem cell training course and teach you exactly what equipment you will need to perform some of the techniques.
- Published in Corporate News / Blog
(Almost) Everything You Wanted to Know About Stem Cells But Were Afraid to Ask
Stem cells have captured the interest of biology nerds, armchair practitioners and everyday individuals for years. Where exactly do they come from and how do they work? When can I have my torn rotary cuff/bum knee /arthritis /(fill in the blank) treated with stem cells? At Global Stem Cells Group, we are making stem cell treatments for a variety of medical conditions available in the physician’s office and out-patient treatment clinics worldwide, and we’re aiming to make them readily available in the U.S. soon, so hang tight.
So, what are stem cells, you ask?
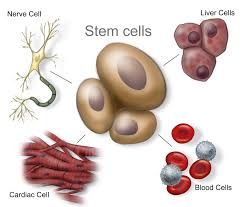 A stem cell is a cell characterized by its ability to self-renew, and its ability to differentiate (change) along multiple pathways. This means a stem cell able is to form specialized cells identical to the cells needed for treatments. For instance, stem cells can become muscle cells, heart cells, skin cells, etc. through stimulation and manipulation via changing chemical environments or genetic triggers. Stem cell physicians know all the tricks to manipulating these uncommitted little units of the body’s rawest materials, and the cells obey!
A stem cell is a cell characterized by its ability to self-renew, and its ability to differentiate (change) along multiple pathways. This means a stem cell able is to form specialized cells identical to the cells needed for treatments. For instance, stem cells can become muscle cells, heart cells, skin cells, etc. through stimulation and manipulation via changing chemical environments or genetic triggers. Stem cell physicians know all the tricks to manipulating these uncommitted little units of the body’s rawest materials, and the cells obey!
So, where to find stem cells when you need them?
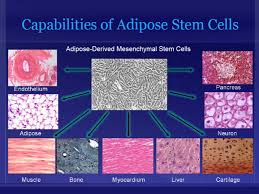 Different types of stem cells come different sources. We like adult adipose-derived stem cells, which are found in abundance in fat—something there seems to be no shortage of! They can be harvested fairly quickly and painlessly from human body fat, which makes them plentiful and readily available. Each liter of fat produces hundreds of millions of potential stem cells. Other stem cells in the body have been examined for their ability to culture usable induced pluripotent stem cells (iPS cells or iPSCs), which are a type of stem cell that can be generated directly from adult cells.
Different types of stem cells come different sources. We like adult adipose-derived stem cells, which are found in abundance in fat—something there seems to be no shortage of! They can be harvested fairly quickly and painlessly from human body fat, which makes them plentiful and readily available. Each liter of fat produces hundreds of millions of potential stem cells. Other stem cells in the body have been examined for their ability to culture usable induced pluripotent stem cells (iPS cells or iPSCs), which are a type of stem cell that can be generated directly from adult cells.
Adult adipose (fat) stem cells appear to be especially prepped for their job, as they are capable of turning into fat, heart, bone or muscle tissue. We know that these fat cells are multipotent, which means they have the ability to self-renew for long periods of time and differentiate into specialized cells with specific functions, thus creating other types of cells.
Today, Global Stem Cells Group has a validated, compliant outpatient method to provide adipose-derived stem cells, as well as bone marrow-derived stem cells, to physicians for use in in-office procedures. We have trained approximately 500 physicians who are currently offering stem cell therapies in their offices and clinics worldwide, and we’ve only scratched the surface.
So, how exactly do stem cells work?
 The concept of stem cell medicine involves the ability to harness the body’s own healing potential to reverse some of the effects of age, degenerative disease and injuries. In other words, we “tell” the stem cells what your body used to be able to do, or how it used to appear, and we have them replicate those youthful, vigorous cells that have not weathered the years or injuries so well.
The concept of stem cell medicine involves the ability to harness the body’s own healing potential to reverse some of the effects of age, degenerative disease and injuries. In other words, we “tell” the stem cells what your body used to be able to do, or how it used to appear, and we have them replicate those youthful, vigorous cells that have not weathered the years or injuries so well.
We often use stem cell therapy interchangeably with regenerative medicine, but this is just one small component of stem cell therapy. We believe the field is going to continue to evolve and move forward, and we’ll be able to combine different techniques to create a truly regenerative response to treatments in patients. The term “stem cell therapy” will eventually become outdated, like when your parents say “groovy.” or “make me a carbon copy.” Instead we will combine techniques like gene therapy and cell therapy in cancers, biologics, scaffolding and delivery systems.
We have really focused on this future in regenerative medicine, combining cellular and gene therapies for use in patients. Everyone in the field is pretty excited about its future, and we believe that regenerative medicine is going to transform medicine as we know it. This is the next big wave for patients who are searching for solutions to their medical needs, but finding few solutions. It’s the 21st century’s version of the polio vaccine, organ transplant and the discovery of antibiotics all rolled up into one—and then some!
- Published in Corporate News / Blog
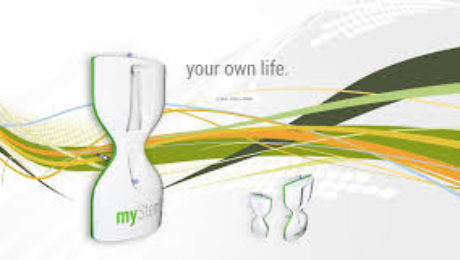
Global Stem Cells Group and Adimarket Announces Agreement with MyStem to Distribute Stem Cell Technology Products in Latin America
Adimarket , a division of Global Stem Cells Group, has signed an agreement to distribute Mystem stem cell technology in Latin America
MIAMI (PRWEB) December 06, 2015
Adimarket, Inc., a leading provider of in-office regenerative medicine solutions, has reached an agreement with MyStem to promote and distribute their stem cell technology products throughout Latin America.
MyStem develops and manufactures regenerative products for use in orthobiologics (ortho, trauma, spine), sports medicine, plastic surgery, aesthetics and dermatology treatments.
MyStem products include the innovative adipose-derived regenerative fraction device MyStem X2, a disposable collection and separation system for autologous adipose-derived mesenchymal stem cells (AdMSC). The MyStem device collects regenerative fraction without any extensive tissue manipulation, according to requirements established under international laws, in a completely safe closed sterile system. The regenerative fraction separation process is based on cells intrinsic parameters in a liquid fraction according to fluidic laws.
After the separation process, a concentrated regenerative cells fraction suspension is ready for use in a one-step procedure. MyStem offers an enhanced method for isolating and quickly expanding a robust population of mesenchymal stem cells (MSCs) derived from lipoaspirate in less than 30 minutes. This isolation process yields a generous population of ASCs (~100,000 cells per 100 ml of blood/saline collected from sonicated lipoaspirate) with differentiation potential, characteristic cell surface markers, and proliferative lifespan indistinguishable from MSCs extracted from bone marrow (BMSCs) or conventionally processed ASCs.(1,2 ).
The MyStem process is simple, with no centrifuge needed. Quality sampling is not operator dependent, and collects up to 1,000 times more than the bone marrow MSC + growth factors.
MyStem Managing Director Pier Ivona calls the new alliance with Adimarket a natural fit, since both companies share a deep commitment to getting critical regenerative medicine tools to the clinicians in Latin America who need them.
“Global Stem Cells Group and MyStem are both pioneers in stem cell research and innovation, and we share the same goal of making stem cell therapies available globally,” Ivona says. “We believe that this is the perfect time to team with Global Stem Cells Group and its Adimarket division to distribute MyStem technology throughout Latin America’s fast-growing medical community.”
The collaboration between Global Stem Cells Group and MyStem reflects one more step toward GSCG’s commitment to expanding its presence in communities that need and deserve access to cutting-edge regenerative medicine, not only in Latin America but also worldwide.
For more information, visit the Adimarket website, email info(at)adimarket(dot)net, or call ![]() +1 305 560 5337.
+1 305 560 5337.
About Adimarket:
Adimarket, Inc., a division of the Global Stem Cells Group, is a cost-competitive online marketplace for quality regenerative medicine equipment and supplies for physicians and health care professionals.
Adimarket was founded to provide physicians and other health care professionals the tools they need to practice regenerative medicine in a medical office setting. Motivated by a firm belief in the impact the practice of stem cell medicine can have when dispensed in a doctor’s office, Adimarket provides physicians with the tools they need to provide patients with cutting edge treatments.
 Adimarket’s experienced customer service representatives provide valuable guidance and advice regarding products relevant to individual practices.
Adimarket’s experienced customer service representatives provide valuable guidance and advice regarding products relevant to individual practices.
About the Global Stem Cell Group:
Global Stem Cells Group, Inc. is the parent company of six wholly owned operating companies dedicated entirely to stem cell research, training, products and solutions. Founded in 2012, the company combines dedicated researchers, physician and patient educators and solution providers with the shared goal of meeting the growing worldwide need for leading edge stem cell treatments and solutions. With a singular focus on this exciting new area of medical research, Global Stem Cells Group and its subsidiaries are uniquely positioned to become global leaders in cellular medicine.
Global Stem Cells Group’s corporate mission is to make the promise of stem cell medicine a reality for patients around the world. With each of GSCG’s six operating companies focused on a separate research-based mission, the result is a global network of state-of-the-art stem cell treatments.
About MyStem:
Wilmington, Delaware-based MyStem is dedicated to contributing to human welfare through the design, manufacture, and sale of regenerative medicine instruments or appliances designed to alleviate pain and restore health. MyStem specializes in regenerative medicine treatments for: spine, ortho, sports medicine, burn and wound healing, CMF (cranio maxillo facial), chronic wounds and diabetic foot wounds.
###
- Published in Press Releases
Stemlab.com Founder and Prominent Plastic Surgeon Alfredo Hoyos to Head Global Stem Cells Group faculty
MIAMI, Oct. 5, 2015— Global Stem Cells Group, Inc. has named Stemlab.com founder and prominent plastic surgeon Alfredo Hoyos, M.D. as permanent head of the GSCG Faculty. Hoyos has been an active member of the GSCG team since signing on as an exclusive representative for the stem cell leader’s Colombian territory in September, 2014.
The Global Stem Cells Group / Stem Cell Training Faculty consists of a distinguished board of physicians, all experts in stem cell and regenerative medicine. The faculty reviews, updates and makes decision pertaining to the training programs offered through the Stem Cell Training division of Global Stem Cells Group. Faculty members also share the latest hand-on techniques and technologies with physicians, nurses, and other health care professionals through SCT’s instructional stem cell training courses.
 Hoyos divides his time between practices in Miami and Bogota, Colombia.
Hoyos divides his time between practices in Miami and Bogota, Colombia.
Hoyos earned his medical degree at the University of Rosario and completed his residency in plastic surgery at Rosario – Saint Joseph Hospital in Bogotá, Colombia. He later founded Stemlab to further the development of aesthetic techniques using stem cell (stromal) derived from adipose tissue.
Stemlab is currently conducting extensive research in regenerative medicine in an effort to establish stem cell treatments that can repair damaged tissue in living organisms, using natural biological mechanisms that are proving useful in auto repair processes on damaged tissue.
Hoyos is the inventor of the High Definition Liposculpture (HDL) and Dynamic Definition at Lipoplasty (4D) techniques as well as other advanced techniques that focus on body contouring.
Author of several scientific articles that discuss innovations and new technologies that work on body contour, Hoyos is also the inventor of proprietary tools and applications of plastic surgery devices.
To learn more, visit the Global Stem Cells Group website, email bnovas@steemcellsgroup.com, or call ![]() +1 305 560 5337.
+1 305 560 5337.
About the Global Stem Cell Group:
Global Stem Cells Group, Inc. is the parent company of six wholly owned operating companies dedicated entirely to stem cell research, training, products and solutions. Founded in 2012, the company combines dedicated researchers, physician and patient educators and solution providers with the shared goal of meeting the growing worldwide need for leading edge stem cell treatments and solutions. With a singular focus on this exciting new area of medical research, Global Stem Cells Group and its subsidiaries are uniquely positioned to become global leaders in cellular medicine.
Global Stem Cells Group’s corporate mission is to make the promise of stem cell medicine a reality for patients around the world. With each of GSCG’s six operating companies focused on a separate research-based mission, the result is a global network of state-of-the-art stem cell treatments.
- Published in Press Releases
Global Stem Cells Group, Inc. Announces New Regenerative Medicine Software App StemData to launch in 2016
MIAMI, Sept. 21, 2015—Global Stem Cells Group, in collaboration with medical software company Datamed SA Chile, has announced the plans to launch StemData, the first cloud-based electronic medical record system specifically designed for regenerative medicine practitioners.
The new StemData system will come programmed with the latest point of care regenerative medicine protocols and follow-up procedures to make it easy for stem cell practitioners to document patient progress and gather important data to establish the safety and efficacy of stem cell treatments in an doctor’s office environment. The system app will be marketed exclusively through Global Stem Cells Group and will be provided free of charge to affiliate physicians in the new Stem Cell Center network.

The cloud-based, server-less StemData solution will be accessible via web, iPad, iPhone, Google Glass and Apple Watch. Global Stem Cells Group and Datamed have utilized the development of the latest electronic medical record (EMR) systems to bring intelligent EMR technology to regenerative medicine specialists.
The StemData app will help regenerative medicine physicians increase efficiencies in their medical practices while improving both treatment and business outcomes. The app will intuitively adapt to each individual practitioner’s unique practice preferences.
To learn more, visit the Global Stem Cells Group website, email bnovas(at)regenestem(dot)com, or call ![]() 305-224-1858.
305-224-1858.
About the Global Stem Cells Group:
Global Stem Cells Group, Inc. is the parent company of six wholly owned operating companies dedicated entirely to stem cell research, training, products and solutions. Founded in 2012, the company combines dedicated researchers, physician and patient educators and solution providers with the shared goal of meeting the growing worldwide need for leading edge stem cell treatments and solutions. With a singular focus on this exciting new area of medical research, Global Stem Cells Group and its subsidiaries are uniquely positioned to become global leaders in cellular medicine.
Global Stem Cells Group’s corporate mission is to make the promise of stem cell medicine a reality for patients around the world. With each of GSCG’s six operating companies focused on a separate research-based mission, the result is a global network of state-of-the-art stem cell treatments.
- Published in Press Releases
Global Stem Cells Group, Inc. Announces Strategic Alliance with Cellus
Global Stem Cells Group has announce a new alliance with Santiago, Chile-based Cellus, a stem cell company dedicated to research, development and commercialization of therapeutic services and custom biotech products in regenerative medicine
MIAMI, FLA. (PRWEB) SEPTEMBER 28, 2015
Global Stem Cells Group has announced a new strategic alliance with Cellus to promote clinical research, physician training and patient treatment in Chile. Based in Santiago, Chile, Cellus is a stem cell company and full GMP lab dedicated to research, development and commercialization of therapeutic services and custom biotech products in the field of regenerative medicine.

Cellus’s state of the art medical facility, located at the Santiago Marriot, includes conference rooms with views to its cell culture lab and a full, cutting edge operating room for performing stem cell procedures. Cellus is managed by a multi-disciplinary team with extensive clinical experience and biopharmaceutical production, operating under the highest standards and international requirements for quality and safety. Cellus is continuously working to develop effective and innovative regenerative medicine solutions using autologous cellular therapies and biological products obtained from and administered to each individual patient.
“We are very pleased about our new alliance with Global Stem Cells Group,” says Cellus Commercial Manager Carlos Girardi. “We plan to leverage this alliance to further our clinical research, and to boost physician training here in Chile.
“This is a great opportunity to prove how valuable a collaboration between two international stem cell forces like Cellus and GSCG can be.”
Global Stem Cells Group, Chile CEO Enrique Testart agrees, adding that the new collaboration between the two companies will help GSCG establish itself as the leader in regenerative medicine therapies in Chile.
“Both Cellus and Global Stem Cells Group bring extraordinary insight, knowledge and experience in stem cell medicine to the table, and together we can accomplish great things,” Testart says. “I’m very excited to work with the excellent team at Cellus.”
The collaboration is part of Global Stem Cells Group’s push to highlight Chile’s appeal as a destination for patients seeking stem cell therapies for a variety of conditions, and as part of its ongoing commitment to expand the reach of stem cell treatments throughout Latin America.
To learn more, visit the Global Stem Cells Group website, email bnovas(at)regenestem(dot)com, or call ![]() 305-224-1858.
305-224-1858.
About the Global Stem Cells Group:
Global Stem Cells Group, Inc. is the parent company of six wholly owned operating companies dedicated entirely to stem cell research, training, products and solutions. Founded in 2012, the company combines dedicated researchers, physician and patient educators and solution providers with the shared goal of meeting the growing worldwide need for leading edge stem cell treatments and solutions. With a singular focus on this exciting new area of medical research, Global Stem Cells Group and its subsidiaries are uniquely positioned to become global leaders in cellular medicine.
Global Stem Cells Group’s corporate mission is to make the promise of stem cell medicine a reality for patients around the world. With each of GSCG’s six operating companies focused on a separate research-based mission, the result is a global network of state-of-the-art stem cell treatments.
About Cellus:
Cellus is dedicated to research, development and commercialization of therapeutic services and custom biotech products in the field of regenerative medicine. Their multidisciplinary team of stem cell experts focus on the prevention and reversal of impairment in the skin of patients with traumatic injuries, skin diseases or symptoms of aging.
Their state-of-the-art facility in Santiago features a full laboratory and showcases the company’s extensive clinical expertise and biopharmaceutical production capabilities.
###
- Published in Press Releases
Global Stem Cells Group, Inc. Announces Collaboration with VidaCel Laboratories and Stem Cell Bank, Chile
Global Stem Cells Group announces a new alliance with Santiago, Chile-based VidaCel Laboratories, Latin America’s oldest and largest cryopreserved umbilical cord blood bank.
MIAMI (PRWEB) SEPTEMBER 25, 2015
Global Stem Cells Group has announced a new collaboration with Santiago, Chile-based VidaCell Laboratories, Latin America’s oldest and largest cryopreserved umbilical cord cell bank. The alliance is focused on creating new protocols for cryopreservation of cord blood, cord tissue, adipose tissue and dental pulp stem cells, and the culture and expansion of cell colonies.
The GSCG / VidaCel alliance will provide both companies broader access to the newest stem cell technologies for application in new territories. With Global Stem Cells Group’s growing presence in Latin America, the collaboration with VidaCel will eliminate the potential for significant delays in collecting and processing stem cells, which can result in a loss of cell viability.
According to Global Stem Cells Group CEO Benito Novas, the rules of the National Program for Bone Marrow Donation in the U.S. provide the guidelines used by VidaCel for processing cord blood cells within 24 hours.
“VidaCel’s location in Chile guarantees immediate availability of cells,” Novas says.
“Their process of collecting, processing and preserving stem cells is performed under the strictest standards of biosecurity, and designed to better safeguard the material over a lifetime, which makes them a perfect collaborator for Global Stem Cells Group.”
Tissue stem cells, or mesenchymal stem cells, harvested from umbilical cord blood and tissue, can differentiate and generate cells of different tissues, mainly connective or supportive, such as bone, cartilage or muscle. Studies show that they can also participate in the process of tissue regeneration through other mechanisms such as immunosuppression, production factors and cell stimulators present in tissue or a damaged organ. Tissue stem cells also have characteristics that allow them to adapt fairly easily to the action of the immune system of an individual, which is why they are essential for use in allogeneic procedures in which immunologic compatibility is a factor.
Stem cells harvested from adipose tissue can differentiate into bone, cartilage, tendon and muscle, and can strengthen defensive and regenerative function. Adipose stem cells are found in adipose (fat) tissue.
Stem cells harvested from dental pulp can also differentiate into bone, cartilage, tendon and muscle to reinforce their defensive and regenerative function. These cells are found in the teeth.
Over time, the body’s stem cells succumb to environmental insults, a common occurrence in the aging process. Cryopreserving stem cells from young patients at an early stage means preserving their maximum viability.
To learn more, visit the Global Stem Cells Group website, email bnovas(at)regenestem(dot)com, or call ![]() 305-224-1858.
305-224-1858.
About the Global Stem Cells Group:
Global Stem Cells Group, Inc. is the parent company of six wholly owned operating companies dedicated entirely to stem cell research, training, products and solutions. Founded in 2012, the company combines dedicated researchers, physician and patient educators and solution providers with the shared goal of meeting the growing worldwide need for leading edge stem cell treatments and solutions. With a singular focus on this exciting new area of medical research, Global Stem Cells Group and its subsidiaries are uniquely positioned to become global leaders in cellular medicine.
Global Stem Cells Group’s corporate mission is to make the promise of stem cell medicine a reality for patients around the world. With each of GSCG’s six operating companies focused on a separate research-based mission, the result is a global network of state-of-the-art stem cell treatments.
About VidaCel:
Established in 2004,VidaCel® is the largest cell blood bank in Latin America, known for its cryopreservation of umbilical cord stem cells, adipose tissue stem cells and dental pulp stem cells. VidaCel follows the rules of the National Program for Bone Marrow Donation’s. U.S. entity, which also provides guidelines concerning Cord Blood Banks.
Located in Santiago, Chile, VidaCel is the first and only public access stem cell bank in Chile, and guarantees immediate availability of cells. VidaCel is committed to the advancement of new technologies and treatments, in conjunction with leading universities. VidaCel is accredited by the Ministry of Health and the Public Health Institute, and all processes are certified by the international quality standard ISO-9001.
- Published in Press Releases
Global Stem Cells Group, Inc. to Launch Two New Editions of “Diplomat in Cell Therapy and Tissue Engineering” Post-Graduate Diploma Program
Global Stem Cells Group will launch two new versions of its “Diplomat in Cell Therapy and Tissue Engineering” post-graduate program. The program will be offered in Chile in 2016
MIAMI (PRWEB) SEPTEMBER 29, 2015
Stem Cell Training, Inc., a division of the Global Stem Cells Group, has announced plans to launch two new editions of the post-graduate diploma program, “Diplomat in Cell Therapy and Tissue Engineering.” The first of its kind worldwide, the program is designed for physicians and qualified practitioners to bring stem cell therapies into the doctor’s office to treat patients.
The program focuses on advances in cell biology that emerged in the late 20th and early 21st centuries to give rise to stem cell therapies—a new form of medical treatment in which cells and tissues are used as healing elements, not only to supplement or replace deficient cells, but to induce regeneration and restoration of a lost biological order during the development of a disease or injury.
According to Global Stem Cells Group CEO Benito Novas, cell therapy is becoming the foundation of future medicine. For that reason, making stem cell sciences and treatment courses available to physicians and qualified medical practitioners is fundamental to the future of medicine. “Diplomat in Cell Therapy and Tissue Engineering” offers professionals invaluable lessons in the new art of healing, as well as the scientific and practical methodologies concerning this new discipline.
The aim of this postgraduate course is to train high-achieving, academic level professionals in cell therapy and tissue engineering to use in different areas of medicine and dentistry.
Course topics include:
- Somatic cell therapy and tissue engineering and associated bioethical issues.
- Cell Structure and functions, cell and tissue growth, cell differentiation and genetic and epigenetic regulation.
- Interaction of cells with other cells and extracellular matrix, humeral microenvironment.
- Development of cell interactions during embryogenesis, tissue regeneration, tissue repair and cancer.
- Immunology: Humeral and cellular immunity, innate and acquired immunity, interactions, effector function, immune tolerance, trophic and reconstructive function.
- Biology of embryo development, and normal and pathological tissue repair: overview of embryonic and fetal post-natal development, genetic and epigenetic factors that influence growth, embryonic fetal and postnatal development, maintenance and repair process of cells, tissue and organs, and genetic and epigenetic factors that interact and influence these processes.
- Identification of certain cellular therapies that repair or correct defects.
- Method of production, processing and application of biological drugs for Cell Therapy and Tissue Engineering.
- Sources of the cells used in cell therapy. Enforcement of cell techniques. Complex manipulation processes, GMP Standards. Cell processing laboratories. The Biosafety and quality of cellular products: Identity, Purity, Power, Security.
- Cell administration. Implant techniques. Evaluating the results of cell therapy.
- Layout of clinical trials.
 Educational strategies will be taught in theory and in practical hands-on classes, during which students can raise questions and work on problem solving. Practical work will be first carried out on animals; laboratory practices will be taught, followed by demonstration of therapies on humans.
Educational strategies will be taught in theory and in practical hands-on classes, during which students can raise questions and work on problem solving. Practical work will be first carried out on animals; laboratory practices will be taught, followed by demonstration of therapies on humans.
Students will take a written exam on the last day; A score of 7 or better out of 10 is required to pass. Evaluations will be complemented with the development of a thesis, to be graded as pass or fail and must be supported with an oral examination.
To learn more, visit the Stem Cells Training website, the Global Stem Cells Group website, email bnovas(at)regenestem(dot)com, or call ![]() 305-224-1858.
305-224-1858.
About the Global Stem Cells Group:
Global Stem Cells Group, Inc. is the parent company of six wholly owned operating companies dedicated entirely to stem cell research, training, products and solutions. Founded in 2012, the company combines dedicated researchers, physician and patient educators and solution providers with the shared goal of meeting the growing worldwide need for leading edge stem cell treatments and solutions. With a singular focus on this exciting new area of medical research, Global Stem Cells Group and its subsidiaries are uniquely positioned to become global leaders in cellular medicine.
Global Stem Cells Group’s corporate mission is to make the promise of stem cell medicine a reality for patients around the world. With each of GSCG’s six operating companies focused on a separate research-based mission, the result is a global network of state-of-the-art stem cell treatments.
About Stem Cell Training, Inc.:
Stem Cell Training, Inc. is a multi-disciplinary company offering coursework and training in 35 cities worldwide. Coursework offered focuses on minimally invasive techniques for harvesting stem cells from adipose tissue, bone marrow and platelet-rich plasma. By equipping physicians with these techniques, the goal is to enable them to return to their practices, better able to apply these techniques in patient treatments.
The company’s training courses are designed to make the best use of stem cell technology available to treat various diseases in a manner that is accessible to everyone. Stem Cell Training, Inc.’s mission is to introduce the promising world of cellular medicine to everyone who can benefit from its application, and to provide high quality, effective and efficient training that complies with the highest medical standards to physicians worldwide.
- Published in Press Releases
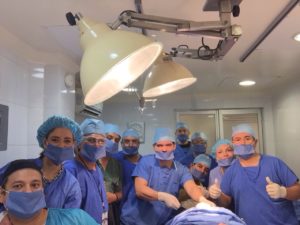
Global Stem Cells Group in Final Stages of Development of Closed System to Isolate Stem Cells
MIAMI (PRWEB) NOVEMBER 30, 2015
Global Stem Cells Group today announced the opening of a new, Good Manufacturing Practice (GMP) 10000 in the Santiago Marriott. The Global Stem Cells Group GMP facility is equipped with the most advanced technologies available, and is operated by a world-class team of qualified medical researchers and practitioners, experienced in administering stem cell protocols using highly manipulated cells.
GMPs are critical for cell therapy success. Understanding and incorporating GMP standards in clinical and pre-clinical research settings establishes a solid foundation on which to build clinical trials and to ensure the safety of biologic products. Good manufacturing practices ensure the safety and efficiency of stem cell products and the patients who receive them. The fundamentals of GMCs are also becoming an important part of the training received by academic researchers in the biotechnology industry, who are at the forefront of developing products for eventual clinical use.

The Global Stem Cells Group GMP 1000 facility will begin receiving international patients in early January 2016.
The GSCG therapeutic center in Santiago—a separate facility from the CMP 1000—will begin seeing international patients as of Dec. 1, 2015 to make its stem cell therapies and facilities available to international patients as well.
For more information, visit the Global Stem Cells Group website, Email bnovas(at)stemcellsgroup(dot)com, or call ![]() 305-560-5337.
305-560-5337.
About the Global Stem Cells Group:
Global Stem Cells Group, Inc. is the parent company of six wholly owned operating companies dedicated entirely to stem cell research, training, products and solutions. Founded in 2012, the company combines dedicated researchers, physician and patient educators and solution providers with the shared goal of meeting the growing worldwide need for leading edge stem cell treatments and solutions.
With a singular focus on this exciting new area of medical research, Global Stem Cells Group and its subsidiaries are uniquely positioned to become global leaders in cellular medicine.
Global Stem Cells Groups corporate mission is to make the promise of stem cell medicine a reality for patients around the world. With each of GSCGs six operating companies focused on a separate research-based mission, the result is a global network of state-of-the-art stem cell treatments.
###
- Published in Press Releases