Stem Cell Researchers Discover Stem Cells That Might Repair Skull, Face Bones
Scientists may be one step closer to a breakthrough that uses stem cells to replace damaged skull and facial bones in patients who experience a head trauma or undergo cancer surgery requiring repair and reconstructive surgery.
Researchers have discovered and isolated stem cells capable of repairing these bones in mice. The research, led by Takamitsu Maruyama and the research team at the University of Rochester Medical Center in Rochester, N.Y., could also help patients born with a skull deformity known as craniosynostosis, which can lead to developmental delays and pressure on the brain.
 In the study, scientists investigated the role of the Axin2 gene in bone formation and regeneration. They also examined a specific mutation that causes craniosynostosis in mice. Their finding show that stem cells involved in skull formation are contained within this cell population. These cells are specificto the bones in the head and are very different from other stem cells involved in the formation of the bones in the legs and other parts of the body.
In the study, scientists investigated the role of the Axin2 gene in bone formation and regeneration. They also examined a specific mutation that causes craniosynostosis in mice. Their finding show that stem cells involved in skull formation are contained within this cell population. These cells are specificto the bones in the head and are very different from other stem cells involved in the formation of the bones in the legs and other parts of the body.
Tests to uncover these cells could also help physicians detect bone diseases caused by stem cell abnormalities, according to the researchers.
The research was published Feb. 1 in the journal Nature Communications.
- Published in Corporate News / Blog
German Stem Cell Scientists Develop 3-D “Mini-retinas” –New Hope for Restoring Sight in Patients with Retinal Degeneration Caused by Diabetes and Inherited Disorders.
Medical breakthroughs using stem cells are aimed at all parts of the body bones, kidneys, joints, spines–and now, sight.
A German study in March in Stem Cell Reports, reports that scientists have created an efficient way of developing 3D retina organoids leverage the self-organizing properties of stem cells to create diverse multi-cellular tissue proxies.
3-D Mini-retinas protocol
The new mini-retina protocol involves cutting an organoid grown from stem cells into three, half-moon shaped pieces at an early stage of eye development. Each of these pieces eventually grows into the full suite of cells found in the retina.
3-D retinal organoids developed in this process efficiently replicate retina formation. This includes the light-detecting cone cells, which now can be produced in high quantities.
cone cells, which now can be produced in high quantities.
Cone photoreceptors, which are responsible for high acuity and color vision, are the most precious retinal cell type with regard to potential future cell replacement therapies in patients affected by retinal degeneration caused by diabetes and inherited disorders.
The process of developing 3D retinal organoids also allows the surviving organoids to grow to reach sizes similar to uncut organoids. These mini-retinas swim around in the dish and because they’re not attached to a surface, better reflect the structure of retinal tissue during development.
In the past, the inability to produce such cells has been a major limitation for regenerative medicine; however, this new method increases the yield of retinal organoids 4-fold, allowing researchers to take a great step forward in the study of the retina and how to repair it.
3-D Mini-retinas offer more diverse ways to study retina tissue
 “The goal isn’t just to make the closest thing next to a real retina, but also to possibly harness the flexibility of the system to create more diverse ways of studying retina tissue,” says senior author Mike Karl, of the German Center for Neurodegenerative Diseases (DZNE) and part of the Center for Regenerative Therapies (CRTD) at Technische Universität Dresden.
“The goal isn’t just to make the closest thing next to a real retina, but also to possibly harness the flexibility of the system to create more diverse ways of studying retina tissue,” says senior author Mike Karl, of the German Center for Neurodegenerative Diseases (DZNE) and part of the Center for Regenerative Therapies (CRTD) at Technische Universität Dresden.
“Even with our new additions to existing organoid systems, we have not yet reached that tipping point of robustness that we need for people without the expertise to grow these models.”
Karl and his colleagues’ comparative studies on pluripotent stem cell-derived human and mouse retina organoids and mouse retina in vivo support the power of the new organoid protocol.
New insights in the study of retinal disease
“Tissue heterogeneity (diversity) is a major challenge in organoid systems, and here our work provides new insight, which will help to develop specific organoid-based models, specifically to reliably study retinal disease mechanism,” says Karl.
The Karl Lab’s change to the mini-retina protocol involves cutting a retina organoid grown from stem cells into three pieces at an early stage of eye development. Each of these pieces, which look like little half moons, eventually grows into the full suite of cells found in the retina, thereby increasing the yield of retinal organoids up to 4-fold compared to previous protocols. Karl’s next objective is to make his 3-D “mini-retinas” even more complex, perhaps by bringing in blood vessels and using the organoids to study regeneration and the function of different neural cell types–specifically, from the human retina.
- Published in Corporate News / Blog
Stem Cell Treatments Normally Used for Cancer Patients are Helping Multiple Sclerosis Patients
The British Broadcasting Corporation (BBC) recently reported that stem cell transplant treatments normally used for cancer patients are helping Multiple Sclerosis (MS) patients in the UK. According to the January 18, 2016 report, 20 patients received bone marrow stem cell transplants using their own stem cells, and that at least some of the patients who were paralyzed by MS are able to walk again post-treatment.
Approximately100,000 people in the United Kingdom suffer from MS, with most new patients diagnosed between the ages of 20 and 30 years of age.
“To have a treatment which can potentially reverse disability is really a major achievement,” says Prof Basil Sharrack, of Sheffield’s Royal Hallamshire Hospital in Sheffield, England.
The treatment, known as autologous hematopoietic stem cell transplantation (HSCT), involves the intravenous infusion of autologous or allogeneic stem cells harvested from the patient’s own bone marrow to reestablish hematopoietic function (formation of blood or blood cells) in patients whose bone marrow or immune system is damaged or defective by chemotherapy. Using stem cells harvested from the patient’s bone marrow helps rebuild the immune system. The theory is that these newly harvested cells are at such an early stage in development that the cellular defects that result in MS do not exist.
“The immune system is being reset or rebooted back to a time point before it caused MS,” says Prof John Snowden, consultant hematologist at Royal Hallamshire Hospital.
The BBC’s Panorama program spoke to several MS patients who have undergone the stem cell transplant.
Steven Storey was diagnosed with MS in 2013 and, within a year, went from being an able-bodied athlete to wheelchair dependent and losing sensation in much of his body.
“I went from running marathons to needing 24-hour acute care. At one point I couldn’t even hold a spoon and feed myself,” Storey says.
Within a few days of the transplant Storey was able to move his toes, and after four months he could stand unaided.
While Storey still needs a wheelchair for mobility, he calls his progress astounding.
“It’s been incredible,” he says. “I was in a dire place, but now I can swim and cycle and I am determined to walk.”
The Royal Hallamshire Hospital along with hospitals in the United States, Sweden and Brazil, is part of an international clinical trial called MIST that is assessing the long-term benefits of the stem cell procedure on MS patients. Study participants all have relapsing remitting MS (RRMS), and received intensive chemotherapy to completely destroy the patients’ immune systems.
Treatment costs are about the same as the annual cost for existing treatments, and the stem cell treatment does not require the use of new or existing medications.
Prof Richard Burt of Northwestern University in Chicago carried out the first hematopoietic stem cell transplantation for MS in 1995, and is coordinating this current MIST international trial, which began in 2006.
“There has been resistance to this in the pharma and academic world,” Burt says. “This is not a technology you can patent and we have achieved this without industry backing.”
A study published last year involving MS patients in Chicago showed significant reductions in neurological disability, and for some the improvements persisted for at least four years, although there was no comparative control group.
The outcomes of the current international trial will be reported in 2018, and may determine whether the stem cell transplant becomes a standard in the United Kingdoms health care system for many MS patients.
“Ongoing research suggests stem cell treatments such as HSCT could offer hope, and it’s clear that in the cases highlighted by Panorama they’ve had a life-changing impact,” says Emma Gray, M.D., head of clinical trials at UK’s MS Society.
- Published in Corporate News / Blog
Global Stem Cells Group Plans Bone Marrow Clinical Trials for Knee Osteoarthritis
Global Stem Cells Group has announced plans to hold clinical trials, pending IRB approval, for bone marrow stem cell treatments targeting knee osteoarthritis. The trials will be held in five GSCG facilities in the U.S. and South America, with 25 patients accepted for each location.
MIAMI, March 31, 2016—Pending Institutional Review Board (IRB) approval, Global Stem Cells Group, Inc. has announced plans to conduct a multi-center, placebo controlled clinical trial to measure the safety and effectiveness of the intra-articular application of freshly isolated bone marrow stem cells for the treatment of osteoarthritis.
The clinical trials, which will begin July 1, 2016 and run for one year, will be held in Global Stem Cell Group facilities in Buenos Aires, Argentina; Bogota, Colombia; Quito, Ecuador; Miami, Florida and Topeka, Kansas. Each center will accept 25 patients per clinical trial, and patients will receive a bone marrow stem cell injection in one knee and a placebo in the other knee..
 The trials are designed to investigate the possible beneficial effect of freshly harvested bone marrow stem cell applications on knee osteoarthritis patients in the control group. Patients will receive standard treatment of bone marrow stem cells intravenously, and will be monitored and assessed for any changes in clinical condition.
The trials are designed to investigate the possible beneficial effect of freshly harvested bone marrow stem cell applications on knee osteoarthritis patients in the control group. Patients will receive standard treatment of bone marrow stem cells intravenously, and will be monitored and assessed for any changes in clinical condition.
Knee osteoarthritis is a chronic, progressive condition affecting an increasing number of people, especially the elderly and obese. It is characterized by degeneration of the cartilage—the natural cushioning between joints inside the knee.
The condition is the result of the wearing away of cartilage. When this happens, the bones of the joints rub more closely against one another with less of the shock-absorbing benefits of cartilage, resulting in pain, swelling, stiffness and a decreased ability to move.
 According to the Centers for Disease Control (CDC), knee osteoarthritis will affect 67 million people in the United States by 2030. While conventional treatments like physiotherapy or drugs offer temporary relief of clinical symptoms, total knee replacement is the closest treatment available for permanent relief, which requires invasive surgery, comes at a high cost and is not always successful. The latest advances in stem cell therapies for knee osteoarthritis are designed to restore cartilage function in the knee.
According to the Centers for Disease Control (CDC), knee osteoarthritis will affect 67 million people in the United States by 2030. While conventional treatments like physiotherapy or drugs offer temporary relief of clinical symptoms, total knee replacement is the closest treatment available for permanent relief, which requires invasive surgery, comes at a high cost and is not always successful. The latest advances in stem cell therapies for knee osteoarthritis are designed to restore cartilage function in the knee.
Global Stem Cells Group offers the most advanced protocols and techniques in cellular medicine from around the world.
Details of the protocol and eligibility criteria will be released upon IRB approval.
For more information on Global Stems Cell Group, visit the Global Stem Cells Group website, email bnovas(at)stemcellsgroup(dot)com, or call +1 305 560 5337.
About Global Stem Cells Group:
Global Stem Cells Group, Inc, is the parent company of six wholly owned operating companies dedicated entirely to stem cell research, training, products and solutions. Founded in 2012, the company combines dedicated researchers, physician and patient educators and solution providers with the shared goal of meeting the growing worldwide need for leading edge stem cell treatments and solutions. With a singular focus on this exciting new area of medical research, Global Stem Cells Group and its subsidiaries are uniquely positioned to become global leaders in cellular medicine.
Global Stem Cells Group’s corporate mission is to make the promise of stem cell medicine a reality for patients around the world. With each of GSCG’s six operating companies focused on a separate research-based mission, the result is a global network of state-of-the-art stem cell treatments.
To view this press release live online, click here
###
- Published in Press Releases
Global Stem Cells Group Announces Manufacturing Phase of Progenikine™ SVF Closed System
Global Stem Cells Group has begun the manufacturing phase of Progenikine™, a new SVF closed system kit utilizing EmCyte technology, containing all the elements necessary to process adipose tissue and obtain stromal vascular fraction in a sterile environment.
MIAMI, March 31, 2016—Global Stem Cells Group, Inc. has announced that Progenikine™, its new and approved SVF closed system kit using EmCyte technology, is in the manufacturing phase and will be available to physicians in July 2016. The Progenikine kit contains all the elements necessary to process adipose tissue and obtain stromal vascular fraction (SVF) in a closed environment.
Adipose derived stem cells (ASCs) are used by physicians for a variety of indications. Most commonly, ASCs are  isolated at the point of care from lipoaspirate (derived from liposuction) tissue as the stromal vascular fraction (SVF), harvested from the patient and immediately administered to the patient as an injection, or used to enrich fat grafts. Isolation of ASCs from adipose tissue is a relatively simple process performed routinely in cell biology laboratories, but isolation at the point of care for immediate clinical administration requires special methodology to prevent contamination, ensure integrity of the clinical procedure, and comply with regulatory requirements.
isolated at the point of care from lipoaspirate (derived from liposuction) tissue as the stromal vascular fraction (SVF), harvested from the patient and immediately administered to the patient as an injection, or used to enrich fat grafts. Isolation of ASCs from adipose tissue is a relatively simple process performed routinely in cell biology laboratories, but isolation at the point of care for immediate clinical administration requires special methodology to prevent contamination, ensure integrity of the clinical procedure, and comply with regulatory requirements.
Developed in conjunction with Patrick Pennie, Emcyte CEO, and and Maritza Novas Director of Research and Development for Global Stem Cells Group, Progenikine  fuses elements from Emcyte systems with the Global Stem cells Group SVF protocols.The kit can provide a low cost, rapid and simple alternative to traditional methods of isolating ASCs, particularly when smaller quantities are needed.
fuses elements from Emcyte systems with the Global Stem cells Group SVF protocols.The kit can provide a low cost, rapid and simple alternative to traditional methods of isolating ASCs, particularly when smaller quantities are needed.
“The Progenikine kit is the newest product designed to help Global Stem Cells Group’s mission to provide accessible products to our member clients, ensuring that more patients will be able to gain access to stem cell therapies,” says Benito Novas, GSCG CEO.
For more information on Global Stems Cell Group, visit the Global Stem Cells Group website,email bnovas(at)stemcellsgroup(dot)com, or call +1 305 560 5337.
About Global Stem Cells Group:
Global Stem Cells Group, Inc. is the parent company of six wholly owned operating companies dedicated entirely to stem cell research, training, products and solutions. Founded in 2012, the company combines dedicated researchers, physician and patient educators and solution providers with the shared goal of meeting the growing worldwide need for leading edge stem cell treatments and solutions. With a singular focus on this exciting new area of medical research, Global Stem Cells Group and its subsidiaries are uniquely positioned to become global leaders in cellular medicine.
Global Stem Cells Group’s corporate mission is to make the promise of stem cell medicine a reality for patients around the world. With each of GSCG’s six operating companies focused on a separate research-based mission, the result is a global network of state-of-the-art stem cell treatments.
About Emcyte:
Fort Myers, Florida-based EmCyte Corporation is a leader in autologous cellular biologics with the GenesisCS Component Concentrating Systems. These systems provide patients with the best opportunity for rapid recovery and provide practitioners with the most advanced clinical point of care experience. EmCyte systems are developed to meet every clinical requirement, giving the physician better clinical choices. EmCyte devices have been independently reviewed and show to produce buffycoat concentrations of 6x to greater than 10x baseline in 7mLs, with yields ranging from 70 percent to greater than 90 percent
EmCyte technology allows for the safe extraction of concentrated platelets and other regenerative cell types from the patient’s own blood. These cells are then re-suspended in a small volume of the patient’s blood plasma and then applied to the treatment site.
###
To view this press release live online, click here
- Published in Press Releases
Our Friend MSCs (Mesenchymal Stem Cells)—Bringing New Life to Old Bones
Researchers from the University of Toronto and The Ottawa Hospital were looking to see if mesenchymal stem cells (MSCs) might treat osteoporosis. MSCs are multipotent stromal cells that can differentiate into a variety of cell types, including: bone cells (osteoblasts), cartilage cells (chondrocytes), muscle cells (myocytes) and fat cells (adipocytes).
Faulty MSCs are the culprits behind osteoporosis; after injecting healthy MSCs into mice with the affliction that causes bones to become weak and brittle, researchers were hoping for a general increase in the mice’s bone health. Instead, they were surprised (and probably very excited) to discover after six months—a quarter of a mouse’s life span—that healthy, functioning bone had replaced the damaged osteoporotic bone. The bone structure in the little creatures, which had been severely compromised by osteoporosis, had been restored to a normal, healthy state! The healthy mesenchymal stem cells did what they were born to do. They became bone cells and went to work, much like the restoration of an old building at the hands of architects and laborers, only without the scaffolds and noise. MSCs work very quietly.
Researchers are hoping that these findings could lead to a new way of treating osteoporosis in humans, or even delay its onset indefinitely.
Stem cell researchers have known for some time that MSCs can boost the regeneration of bone, and in fact a test group of elderly patients in the U.S. who suffer from osteoporosis have already received MSC injections as part of an ancillary trial. The research team is preparing to to examine their blood samples to see if biological markers indicate an improvement in bone growth and bone reabsorption.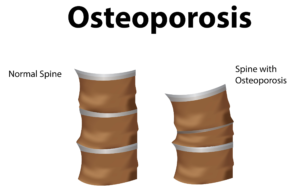
Depending on the outcome of those blood tests, larger trials involving human patients could follow within the next 5 years.
In addition to working quietly and therefore not waking you to the sound of a jackhammer at 7 a.m., there are other cool qualities to MSCs. For instance, they are “a heterogeneous population of musculoskeletal progenitors (another name for adult stem cells) that includes skeletal stem cells (SSCs).” An added perk is that they can be transplanted between individuals without the need to be matched, and without the risk of rejection.
MSCs are awesome.
Globally, more than 200 million people are living with either postmenopausal osteoporosis—known as type 1 osteoporosis, which affects mainly women, or age-related type 2 osteoporosis, which affects both men and women.
With type 2 osteo porosis, there is a reduction in the inner structure of the bone. The bone becomes thinner and less dense, and it can no longer function properly.
porosis, there is a reduction in the inner structure of the bone. The bone becomes thinner and less dense, and it can no longer function properly.
Worldwide, type 2 osteoporosis leads to around 8.9 million bone fractures annually. Hip fractures are among the most common fractures related to osteoporosis, which can lead to disability and even death in elderly patients.
Currently, Teriparatide (brand name Fortéo) is the only drug available to treat type 2 osteoporosis, and its effectiveness lasts for only two years.
The senior author of the study, titled Systemic Mesenchymal Stromal Cell Transplantation Prevents Functional Bone Loss in a Mouse Model of Age-Related Osteoporosis, and published March 17, 2016, is William Stanford, Ph.D., a senior scientist at The Ottawa Hospital Research Institute and a professor at the University of Ottawa. Previous research led Stanford to discover the association between defects in MSC and age-related osteoporosis in mice.
The study’s co-author, John E. Davies, Ph.D., D.Sc., is a professor at the University of Toronto’s Institute of Biomaterials and Biomedical Engineering, The study’s findings are published in the current issue of Stem Cells Translational Medicine.
- Published in Corporate News / Blog
How Stem Cell Therapies Can Help Heal Sports Injuries
Continuing our recent discussion of stem cell therapies for sports injuries, the use of mesanchysmal stem cells (MSCs) in orthopedic medicine can help in the repair of damaged tissue by harnessing the healing power of undifferentiated cells that form all other cells in our bodies. The process involves isolating these stem cells from a sample of your blood, bone marrow or adipose tissue (fat cells), and injecting it into the damaged body part to promote healing. Platelet-rich-plasma (PRP), a concentrated suspension of platelets (blood cells that cause clotting of blood) and growth factors, is also used to assist the process of repair.
Below are some examples of injuries and areas of research involving the use of mesenchymal stem cells (MSCs), which are (adult) tissue stem cells that are not only able to produce copies of themselves, but also able to divide and form bone, cartilage, muscle, and adipose (fat) stem cells when cultured under certain conditions:
Cartilage Damage
Cartilage has long bee n considered as an ideal candidate for cell therapy as it is a relatively simple tissue, composed of one cell type, chondrocytes, and does not have a substantial blood-supply network. Of particular interest to researchers is repair of cartilage tissue in the knee, also called the meniscus of the knee. The meniscus is responsible for distributing the body’s weight at the knee joint when there is movement between the upper and lower leg. Only one third of meniscus cartilage has a blood supply, and as the blood supply allows healing factors and stem cells attached to the blood vessels (called perivascular stem cells) to access the damaged site, the meniscus’s natural lack of blood supply impairs healing of this tissue. Damage to this tissue is common in athletes, and is the target for surgery in 60 percent of patients undergoing knee operations, which usually involves the partial or complete removal of the meniscus, which can lead to long-term cartilage degeneration and osteoarthritis.
n considered as an ideal candidate for cell therapy as it is a relatively simple tissue, composed of one cell type, chondrocytes, and does not have a substantial blood-supply network. Of particular interest to researchers is repair of cartilage tissue in the knee, also called the meniscus of the knee. The meniscus is responsible for distributing the body’s weight at the knee joint when there is movement between the upper and lower leg. Only one third of meniscus cartilage has a blood supply, and as the blood supply allows healing factors and stem cells attached to the blood vessels (called perivascular stem cells) to access the damaged site, the meniscus’s natural lack of blood supply impairs healing of this tissue. Damage to this tissue is common in athletes, and is the target for surgery in 60 percent of patients undergoing knee operations, which usually involves the partial or complete removal of the meniscus, which can lead to long-term cartilage degeneration and osteoarthritis.
Recently, researchers increased their focus on the use of MSCs for treatment of cartilage damage in the knee. Some data from animal models suggest that damaged cartilage undergoes healing more efficiently when MSCs are injected into the injury, and this can be further enhanced if the MSCs are modified to produce growth factors associated with cartilage. It has been shown that once the MSCs are injected into the knee they attach themselves to the site of damage and begin to change into chondrocytes, promoting healing and repair. A small number of completed clinical trials in humans using MSCs to treat cartilage damage have reported some encouraging results, but these studies used very few patients, making it difficult to accurately interpret the results. There are currently a number of ongoing trials using larger groups of patients, and the hope is that these will provide more definite information about the role MSCs play in cartilage repair.
Tendinopathy
Tendinopathy relates to injuries that affect tendons – the long fibrous tissues that connect and transmit force from  muscles to bones. Tendons become strained and damaged through repetitive use, making tendinopathy a common injury among athletes. Tendinopathy has been linked to 30 percent of all running-related injuries, and up to 40 percent of tennis players suffer from some form of elbow tendinopathy or “tennis elbow.” Damage occurs to the collagen fibers that make up the tendon, and this damage is repaired by the body through a process of inflammation and production of new fibers that fuse together with the undamaged tissue. However, this natural healing process can take up to a year to resolve, and results in the formation of a scar on the tendon tissue, reducing the tendon’s natural elasticity, decreasing the amount of energy the tissue can store and resulting in a weakening of tendon.
muscles to bones. Tendons become strained and damaged through repetitive use, making tendinopathy a common injury among athletes. Tendinopathy has been linked to 30 percent of all running-related injuries, and up to 40 percent of tennis players suffer from some form of elbow tendinopathy or “tennis elbow.” Damage occurs to the collagen fibers that make up the tendon, and this damage is repaired by the body through a process of inflammation and production of new fibers that fuse together with the undamaged tissue. However, this natural healing process can take up to a year to resolve, and results in the formation of a scar on the tendon tissue, reducing the tendon’s natural elasticity, decreasing the amount of energy the tissue can store and resulting in a weakening of tendon.
MSCs have the ability to generate cells called tenoblasts that mature into tenocytes. These tenocytes are responsible for producing collagen in tendons. This link between MSCs and collagen is the focus for researchers investigating how stem cells may help treat tendinopathy. Substantial research has been carried out on racehorses as they suffer from high rates of tendinopathy, and the injury is similar to that found in humans. Researchers discovered that by injecting MSCs isolated from an injured horse’s own bone marrow into the damaged tendon, recurrence rates were almost cut in half compared to horses that receive traditional medical management for this type of injury. A later study by the same group showed the MSCs improved repair, resulting in reduced stiffness of the tissue, decreased scarring and better fusion of the new fibers with the existing, undamaged tendon. It is not yet clear if these results are due to MSCs producing new tenocytes or their ability to modulate the environment around the tendinopathy, as described above. These promising results paved the way for the first pilot study in humans.
Bone Repair
 Bones are unique in that they have the ability to regenerate throughout life. Upon injury, such as a fracture, a series of events occur to initiate healing of the damaged bone. Initially there is inflammation at the site of injury, and a large number of signals are sent out. These signals attract MSCs, which begin to divide and increase their numbers. The MSCs then change into either chondrocytes, the cells responsible for making a type of cartilage scaffold, or osteoblasts, the cells that deposit the proteins and minerals that comprise the hard bone on to the cartilage. Finally these new structures are altered to restore shape and function to the repaired bone. A number of studies carried out in animals have demonstrated that direct injection or infusing the blood with MSCs can help heal fractures that previously failed to heal naturally. However, as was the case with tendinopathy, it is not yet clear if these external MSCs work by generating more bone-producing cells or through their ability to reduce inflammation and encourage restoration of the blood supply to injured bone, or both.
Bones are unique in that they have the ability to regenerate throughout life. Upon injury, such as a fracture, a series of events occur to initiate healing of the damaged bone. Initially there is inflammation at the site of injury, and a large number of signals are sent out. These signals attract MSCs, which begin to divide and increase their numbers. The MSCs then change into either chondrocytes, the cells responsible for making a type of cartilage scaffold, or osteoblasts, the cells that deposit the proteins and minerals that comprise the hard bone on to the cartilage. Finally these new structures are altered to restore shape and function to the repaired bone. A number of studies carried out in animals have demonstrated that direct injection or infusing the blood with MSCs can help heal fractures that previously failed to heal naturally. However, as was the case with tendinopathy, it is not yet clear if these external MSCs work by generating more bone-producing cells or through their ability to reduce inflammation and encourage restoration of the blood supply to injured bone, or both.
Brain injury in sports
There is mounting evidence that those taking part in sports where they are exposed to repetitive trauma to the head and brain are at a higher risk of developing neurodegenerative disorders, some of which are targets for stem cell treatments. For example, it has been reported that the rate of these diseases, like Alzheimer Disease, were almost four times higher in professional American football players compared to the general population. While the cause of this disease is not yet clear, it is associated with abnormal accumulation of proteins in neural cells that eventually un dergo cell death and patients develop dementia. Researchers have attempted a number of strategies to investigate treatments of this disease in mice, including introducing neural stem cells that could produce healthy neurons. While some of these experiments have demonstrated positive, if limited, effects, to date there are no stem cell treatments available for Alzheimer’s Disease.
dergo cell death and patients develop dementia. Researchers have attempted a number of strategies to investigate treatments of this disease in mice, including introducing neural stem cells that could produce healthy neurons. While some of these experiments have demonstrated positive, if limited, effects, to date there are no stem cell treatments available for Alzheimer’s Disease.
Boxers suffering from dementia pugilistica, a disease thought to result from damage to nerve cells, can also demonstrate some symptoms of Parkinson’s Disease (among others). In healthy brains, specialized nerve cells called dopaminergic neurons produce dopamine, a chemical that transmits signals to the part of the brain responsible for movement. The characteristic tremor and rigidity associated with Parkinson’s Disease is due to the loss of these dopaminergic neurons and the resulting loss of dopamine production. Researchers are able to use stem cells to generate dopaminergic neurons in the lab that are used to study the development and pathology of this disease. While a recent study reported that dopaminergic neurons derived from human embryonic stem cells improved some symptoms of the disease in mice and rats, stem cell based treatments are still in the development phase.
- Published in Corporate News / Blog
Stem cells and Platelet Rich Plasma Therapies Look Promising for Treating a Variety of sports-related injuries
Stem cell therapies for the treatment of various injuries and diseases that afflict athletes and sportspeople have been the focus of researchers for at least a few years, and recent findings are optimistic.
Stem cell scientists worldwide have been actively pursuing stem cell therapies to harness the process by which stem cells repair and replace damaged tissues and cells.
Researchers at the Mayo Clinic in Rochester, Minnesota are also incorporating platelet rich plasma (PRP) harvested from the patient’s own blood to isolate and concentrate platelets (clotting cells) and then inject them back into the injured area to amend inflammation and initiate the healing process. PRP preparations can be individualized to meet the patient’s specific needs.
The Mayo Clinic is also leveraging stem cells’ ability to regenerate tissues to promote healing. For sports injury therapies stem cells are harvested from the patient’s bone marrow, which also contains other potentially therapeutic cells. Stem cells are powerful, naturally occurring cells that have the ability to modify inflammation and promote natural healing. Mayo Clinic researchers obtain bone marrow from the patient’s pelvic bone, concentrate it to remove the unwanted portions, and inject it back into the injured area.
Global Stem Cells’ associate Joseph Purita, M.D., an osteopathic surgeon and pioneer in the use of stem cells and regenerative medicine techniques to treat sports injuries, uses PRP in concert with stem cells. Dr. Purita has been the focus of much attention for his breakthrough stem cell and PRP treatments to treat injured professional athletes, and has been credited for bringing orthopedic stem cell therapies into the worldwide sports injuries spotlight.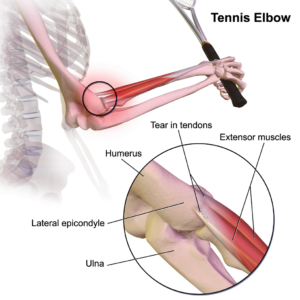
Stem Cell Treatments for Muscle Repair
Forty-five percent of all sports-related injuries involve muscle contusion and strain. Muscle tissue is composed of long, tubular cells called myoblasts—embryonic cells—that fuse to form muscle fibers. Muscle stem cells—also called satellite cells—are responsible for muscle repair. When an individual exercises, muscle fibers become damaged and send signals to these satellite cells, which are perched on top of the muscle tissue. In response to the damaged muscle tissue signals, the satellite cells become activated, begin to divide, replicate themselves and generate new myoblast cells. These myoblasts are then integrated and repair the damaged muscle tissue. Recently, scientists have discovered that satellite cells from older mice are not able to regenerate muscle as efficiently as those from young mice, a discovery that led researchers to identify drugs that restore the function of older cells, which could be used in the future to enhance muscle tissue repair.
Mesenchymal Stem Cells (MSCs) used for injury repair
Mesenchymal stem cells (MSCs) are (adult) tissue stem cells that are not only able to produce copies of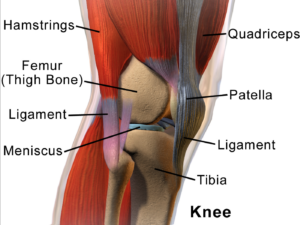 themselves, they can divide and form bone, cartilage, muscle, and adipose (fat) stem cells when cultured under certain conditions. MSCs are attractive to researchers and clinicians since they can be readily isolated from a variety of patient tissues, including fat. Once harvested and, if necessary, elevated to high numbers in culture, MSCs can be re-introduced to the patient from whom they were harvested, eliminating any risk of immune-rejection. In response to injury, MSCs produce proteins that appear to alter the surrounding environment and promote healing and tissue regeneration, such as anti-inflammatory factors, angiogenic factors (which promote the growth of new blood vessels) and other factors that stimulate local, tissue-specific stem cells.
themselves, they can divide and form bone, cartilage, muscle, and adipose (fat) stem cells when cultured under certain conditions. MSCs are attractive to researchers and clinicians since they can be readily isolated from a variety of patient tissues, including fat. Once harvested and, if necessary, elevated to high numbers in culture, MSCs can be re-introduced to the patient from whom they were harvested, eliminating any risk of immune-rejection. In response to injury, MSCs produce proteins that appear to alter the surrounding environment and promote healing and tissue regeneration, such as anti-inflammatory factors, angiogenic factors (which promote the growth of new blood vessels) and other factors that stimulate local, tissue-specific stem cells.
- Published in Corporate News / Blog
Global Stem Cells group to Launch New Clinical Site in Quito, Ecuador with an Inaugural Conference and Stem Cell Training Event
Global Stem Cells Group has announced plans to inaugurate its new stem cell center in Quito, Ecuador with an inaugural conference and stem cell training event Feb. 24-March 6. The new facility will offer the latest stem cell therapies to treat a variety of patient needs.
MIAMI, Feb. 22, 2016—Global Stem Cells Group, has announced an inaugural conference and stem cell training event to launch  its new stem cell treatment in Quito, Ecuador. Feb. 24-March 6, 2016 launch of a new stem cell treatment center in Quito, Ecuador. The new facility will provide advanced protocols and state-of-the-art techniques in cellular medicine to patients from around the world.
its new stem cell treatment in Quito, Ecuador. Feb. 24-March 6, 2016 launch of a new stem cell treatment center in Quito, Ecuador. The new facility will provide advanced protocols and state-of-the-art techniques in cellular medicine to patients from around the world.
Global Stem Cells Group CEO Benito Novas will host the event, which will begin with a stem cell conference featuring a renowned group of stem cell experts who will be featured speakers, including Global Stem Cells Group Chief Operating Officer Kipp Van Camp, D.O., Joseph Purita, M.D., and Pablo Cornejo, M.D. Purita, who heads GSCG’s Stem Cell Training, and Cornejo will perform a stem cell treatment on a high level Ecuadorian government official to address an orthopedic condition. The official cannot be named due to medical privacy standards.

Joseph Purita, M.D.
The opening of the Quito stem cell clinic—the first in Ecuador—is part of Global Stem Cells Group’s expanding presence in Latin America.
“We’re excited to launch the Quito Global Stem Cells Group clinic and establish our services in Ecuador,” says Novas. “The Quito clinical staff of highly qualified and regarded physicians and medical professionals is enthusiastic about their roles as pioneers in bringing stem cell therapies to the country.”

Kipp Van Camp, D.O.
The Quito event will be held at the Hotel Hilton Quito.
Global Stem Cells Group provides stem cell treatments for a variety of conditions and diseases including arthritis, autism, chronic obstructive pulmonary disease (COPD), diabetes, multiple sclerosis, injuries and more at various facilities worldwide. The new facility in Quito will have an

Pablo Cornejo, M.D.
Global Stem Cells Group’s Quito clinic is certified for the medical tourism market, and staff physicians are board-certified or board-eligible. GSCG clinics provide services in more than 10 specialties, attracting patients from the United States and around the world.
The Global Stem Cells Group is committed to providing the highest of standards of services and technology, expert and compassionate care, and a philosophy of exceeding the expectations of their international patients.
For more information, visit the Global Stem Cells Group website, email bnovas(at)stemcellsgroup(dot)com, or call +1 305 560 5337.
About the Global Stem Cell Group:
Global Stem Cells Group, Inc. is the parent company of six wholly owned operating companies dedicated entirely to stem cell research, training, products and solutions. Founded in 2012, the company combines dedicated researchers, physician and patient educators and solution providers with the shared goal of meeting the growing worldwide need for leading edge stem cell treatments and solutions. With a singular focus on this exciting new area of medical research, Global Stem Cells Group and its subsidiaries are uniquely positioned to become global leaders in cellular medicine.
Global Stem Cells Group’s corporate mission is to make the promise of stem cell medicine a reality for patients around the world. With each of GSCG’s six operating companies focused on a separate research-based mission, the result is a global network of state-of-the-art stem cell treatments.
To view this press release live online, click here
###
- Published in Press Releases
Global Stem Cells Group Announces New Clinic Opening in Quito, Ecuador
Global Stem Cells Group has announced the opening of a new stem cell clinic in Quito, Ecuador. The new facility will offer the latest stem cell and regenerative medicine treatments for orthopedic and trauma applications.
MIAMI, Feb. 11, 2016—Global Stem Cells Group, has announced the opening of its new stem cell treatment clinic in Quito, Ecuador. The new facility will provide advanced protocols and state-of-the-art techniques in cellular medicine, focusing on orthopedic and trauma applications to patients from around the world.
Ecuador. The new facility will provide advanced protocols and state-of-the-art techniques in cellular medicine, focusing on orthopedic and trauma applications to patients from around the world.

Pablo Cornejo, MD
The new GSCG clinic is headed by four prominent Ecuadorian physicians, including Pablo Ramos, M.D. an orthopedic specialist and attending physician at Metropolitan Hospital in Quito; Pablo Cornejo, M.D., a specialist in orthopedic and trauma surgery at Metropolitan Hospital, Quito; Luis Ernesto Mantilla, M.D., a specialist in trauma and knee Surgery,at Metropolitan Hospital, Quito, and Pablo Ramos, M.D., a specialist in trauma surgery and arthroscopy at Metropolitan Hospital, Quito.
The opening of the Quito stem cell clinic marks Global Stem Cells Group’s first facility in Ecuador, and is part of GSCG’s expanding presence in Latin America.
[su_spacer size=”10″]

Luis Ernesto Mantilla, MD
Global Stem Cells Group has been expanding its clinical presence worldwide by partnering with qualified physicians experienced in stem cell therapies to open new clinics, licensed and developed under the GSCG banner.
Global Stem Cells Group provides stem cell treatments for a variety of conditions and diseases including arthritis, autism, chronic obstructive pulmonary disease (COPD), diabetes, multiple sclerosis, injuries and more at various facilities worldwide. The new facility in Quito will have an international staff that is experienced in administering the leading cellular therapies available.
Global Stem Cells Group’s Quito clinic is certified for the medical tourism market, and staff physicians are board-certified or board-eligible. GSCG clinics provide services in more than 10 specialties, attracting patients from the United States and around the world.

Pablo Ramos, M.D.
For more information, visit the Global Stem Cells Group website, email bnovas(at)stemcellsgroup(dot)com, or call +1 305 560 5337.
About the Global Stem Cell Group:
Global Stem Cells Group, Inc. is the parent company of six wholly owned operating companies dedicated entirely to stem cell research, training, products and solutions. Founded in 2012, the company combines dedicated researchers, physician and patient educators and solution providers with the shared goal of meeting the growing worldwide need for leading edge stem cell treatments and solutions. With a singular focus on this exciting new area of medical research, Global Stem Cells Group and its subsidiaries are uniquely positioned to become global leaders in cellular medicine.
Global Stem Cells Group’s corporate mission is to make the promise of stem cell medicine a reality for patients around the world. With each of GSCG’s six operating companies focused on a separate research-based mission, the result is a global network of state-of-the-art stem cell treatments.
To view this press release live online, click here
###
- Published in Press Releases