Stem Cell-stimulating Fillings Help Regenerate Teeth Damaged by Disease, Decay
Researchers from Harvard University and the University of Nottingham have developed a new filling that stimulates stem cells in dental pulp to regenerate and even regrow teeth damaged by disease and decay. According to Newsweek Magazine, the discovery earned a prize from the Royal Society of Chemistry after judges described it as a “new paradigm for dental treatments.”
The treatment is believed to potentially eliminate the need for root canals.
Filling materials stimulate stem cells to encourage dentin growth
The filling works by stimulating the body’s natural store of stem cells to encourage the growth of dentin—the bony material that makes up the majority of the tooth—allowing patients to effectively regrow teeth that are damaged through dental disease. The filling’s synthetic biomaterials are used similarly to dental fillings, placed in direct contact with pulp tissue in the damaged tooth. This stimulates the tissue’s native stem cell population to repair and regenerate pulp tissue and the surrounding dentin.
The discovery is a significant step forward from current methods to treat cavities, which involve drilling out decay and putting in a filling made of gold; porcelain; silver amalgam (which consists of mercury mixed with silver, tin, zinc, and copper); or tooth-colored plastic or composite resin. When these fillings fail to halt the tooth’s decay, a root canal is needed to remove the pulp of the tooth, damaging it even further.
Alternative to traditional fillings in teeth
Researchers hope to develop the technique with industry partners in order to make it available for dental patients as an alternative to traditional fillings. Marie Curie research fellow Adam Celiz says that existing dental fillings are toxic to cells and are therefore incompatible with pulp tissue inside the tooth.
“In cases of dental pulp disease and injury, a root canal is typically performed to remove the infected tissues,” Celiz says.
The promise of using therapeutic biomaterials to bring stem cell medicine to restorative dentistry could significantly impact millions of dental patients each year. In fact, the approach is so promising it won second prize in the materials category of the Royal Society of Chemistry’s Emerging Technology Competition for 2016.
Competition entries were judged on the degree of innovation of the technology, its potential impact, and the quality of the science behind it. Increasing innovation in the chemical sciences is a key element of the Royal Society of Chemistry’s industry strategy.
 Effective and practical approach to regenerating teeth
Effective and practical approach to regenerating teeth
The stem cell stimulating filling promises to change the future of dentistry, according to David Mooney, Pinkas Family Professor of Bioengineering at the John Paulson School of Engineering and Applied Sciences at Harvard and the Wyss Institute for Biologically Inspired Engineering.
“’These materials may provide an effective and practical approach to allow a patient to regenerate components of their own teeth,’ Pinkas says.
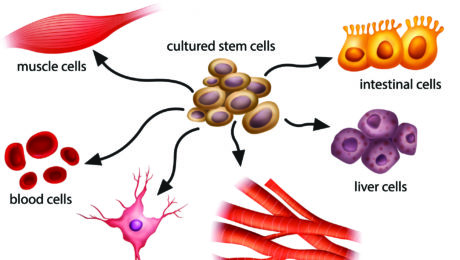
Stem cells can induce regenerative, self-healing qualities in any tissue found in the body and can, as a result, provide unlimited potential for medical applications. Current studies are underway worldwide to learn how stem cells may be used to prevent or cure diseases and injuries such as Parkinson’s disease, type 1 diabetes, heart disease, spinal cord injury, muscular dystrophy, Alzheimer’s disease, strokes, burns, osteoarthritis, vision and hearing loss, and more. Stem cells may also be used to replace or repair tissue damaged by disease or injury.
###
- Published in Corporate News / Blog
New Guidelines for Stem Cell Research and Therapies Aim to Protect Patients from Charlatan Quackery
Stem cell research has never been more advanced, and as a result many different types of treatments are currently offered on the market. Unfortunate
ly, some providers are practicing quackery in stem cell therapies, and an abundance of well-intentioned scientific and medical personnel are prematurely publicizing their work. These providers and publishers have cast an unfair shadow of mistrust on this very important branch of medical research and potential treatments.
On the other hand, the contributions of professional medical and stem cell societies and other organizations require self-regulation through accreditation and certification, development of standards, and creation of a platform for collaboration among stakeholders.
Professional Guidelines for responsible Stem Cell Research
 International Society for Stem Cell Research (ISSCR) is the largest professional organization of stem cell scientists. In 2007, ISSCR impaneled a broad international taskforce to develop a set of professional guidelines for responsible translational stem cell research. Their principles include high standards of preclinical evidence, peer review, scrupulous review of clinical protocol by an Institutional Review Board (IRB), rigorous informed consent, and publication of results whether positive or negative.
International Society for Stem Cell Research (ISSCR) is the largest professional organization of stem cell scientists. In 2007, ISSCR impaneled a broad international taskforce to develop a set of professional guidelines for responsible translational stem cell research. Their principles include high standards of preclinical evidence, peer review, scrupulous review of clinical protocol by an Institutional Review Board (IRB), rigorous informed consent, and publication of results whether positive or negative.
The general scientific consensus is that most stem cell therapies are not ready for marketing or commercialization. But the industries that are providing these treatments are increasingly sophisticated and organized, and are challenging established regulatory frameworks.
The International Society for Cellular Therapy (ISCT) has an interest in the promotion of stem cell research and development, but it also is interested in a broader range of cell-based interventions such as immune cell interventions, reproductive medicine, and gene therapy. The ISCT taskforce has working groups on definitions, scientific evidence and biological rationale, laboratory cell processing, clinical practice, regulation, commercial implications, communications, and policy.
Develop terminology, define levels of scientific evidence in new guidelines for stem cell research
The key goals are to develop an appropriate terminology, define the levels of scientific evidence needed to justify routine use or commercialization of a stem cell therapy, address questions of “experimental” and “innovative” use, and understand the global regulatory landscape in order to identify gaps and contradictions.
The ISSCR published revised guidelines for research and clinical translation involving stem cells on May 12, 2016. These new guidelines update and combine guidelines on stem cell research and clinical translation previously issued in 2006 and 2008 Jonathan Kimmelman, Associate Professor of Biomedical Ethics at McGill University, chaired the ISSCR Guidelines Update Task Force. The task force was made up of 25 experts in basic research, clinical research, and bioethics, and received feedback from 85 external individuals and organizations.
2016 guidelines: covering new ground in stem cell research
The 2016 guidelines cover new ground in areas such as gene editing and induced pluripotent stem cells. They introduce a new focus on the communication of results. The task force recognizes that results and potential applications can be exaggerated, leading to distorted understandings of research outcomes in the scientific community, popular press, and among potential patients. The “14-day rule” limiting experimentation on human embryos or embryo-like structures is upheld in these guidelines, although one task-force member has suggested that this may soon be open to revision.
In May, 2016 ISSCR released the following list of all of the new topics addressed in the revised guidelines as part of the announcement of its report:
- Define an Embryo Research Oversight (EMRO) process to encompass both human embryonic stem cell research and human embryo research that may not explicitly pertain to stem cells or generating new stem cell lines;
- Exclude the generation of induced pluripotent stem cells (iPS cells) from specific stem cell research oversight, and instead call on the existing human subjects review processes to oversee donor cell recruitment (iPS cells behave like embryonic stem cells but are derived by reprogramming more differentiated tissue cells);
- Support laboratory-based research that entails gene editing of the nuclear genomes of human sperm, egg, or embryos, when performed under rigorous review, but hold that any attempt to apply this clinically would be premature and should be prohibited at this time;
- Define principles for evaluating both basic and clinically applied research on mitochondrial replacement therapy, in concordance with recent deliberations in the U.K., U.S., and elsewhere;
- Determine that where there is no undue financial inducement to participate, it may be acceptable to compensate women who donate eggs for research;
- Recognize that the development of increasingly complex in vitro models of early stages of human development should undergo specialized review;
- Highlight opportunities to strengthen preclinical studies in stem cell research, including reproducibility and stringent standards for experimental design;
- Call for robust standards for preclinical and clinical research evidence as clinical trials progress and rigorous evaluation for safety and efficacy before marketing approval;
- Address the valuable contributions made by patients or patient groups to support clinical research and a framework to ensure this is achieved without compromising the integrity of the research;
- Highlight the responsibility of all groups communicating stem cell science and medicine—scientists, clinicians, industry, science communicators, and media—to present accurate, balanced reports of progress and setbacks.
The good news is that stem cell research is evolving into a highly respected and in-demand branch of healing that many  consider to be the future of medicine. Since pluripotent stem cells have the ability to differentiate into any type of cell, they are used in the development of medical treatments for a wide range of conditions including physical trauma, degenerative conditions, and genetic diseases (in combination with gene therapy). Further treatments using stem cells are being developed due to stem cells’ ability to repair extensive tissue damage.
consider to be the future of medicine. Since pluripotent stem cells have the ability to differentiate into any type of cell, they are used in the development of medical treatments for a wide range of conditions including physical trauma, degenerative conditions, and genetic diseases (in combination with gene therapy). Further treatments using stem cells are being developed due to stem cells’ ability to repair extensive tissue damage.
Great levels of success and potential have been achieved from research using adult stem cells. In early 2009, the FDA approved the first human clinical trials using embryonic stem cells. Embryonic stem cells are pluripotent, which means they can become any cell type of the body, with the exception of placental cells. More and more is being discovered about the plasticity of adult stem cells, increasing the potential number of cell types an adult stem cell can become.
###
- Published in Corporate News / Blog
Researchers Move Closer to Lung Stem Cell Therapies to Treat Chronic Lung Diseases
Chronic lung diseases are the third leading causes of death in the U.S. Chronic lung diseases include a collection of illnesses that cause airflow blockage and breathing-related issues, including primarily chronic obstructive pulmonary disease (COPD), bronchitis, emphysema and asthma. Lung disease involves changes in cells within the lungs, and while research on lung stem cell therapies may not only shed light on their causes, it may provide the groundwork for future treatments.
Stem cells in the lung
Human lungs are hard working organs. In an average lifetime, human lungs take 20-40 million breaths and experience a daily airflow of between 1,850 and 2,640 gallons. Human lungs are made up of two distinct regions:
- The conducting airway tubes, including the trachea, bronchi, and bronchioles.
- The gas exchange regions, or alveolar spaces.
Medical researchers have discovered that these regions each contain unique types of stem cells and progenitor cells. In normal lungs, an abundance of progenitor cells is present in each region, which divide to replace old or damaged lung cells to keep the lungs healthy. The progenitor cells include tracheal basal cells, bronchiolar secretory cells (known as club cells), and alveolar type 2 cells. Progenitor cell division is believed to be sufficient to renew the lung’s structure throughout normal adult life.
Stem cells are far less abundant than progenitors, but are found in both embryonic and adult lungs. Some stem cells assist in initial lung development, while others help repair and regenerate the lung throughout one’s lifetime. Problematic stem cells may actually contribute to lung diseases. In mouse lungs, certain rare stem cells have been located in the conducting airway tubes after to severe injury—for example, flu infection. These rare cells can divide and produce new cells that contribute to both the airway and gas exchange regions. These cells have also been grown in vitro and used as a proof-of-concept treatment in injured mouse lungs.
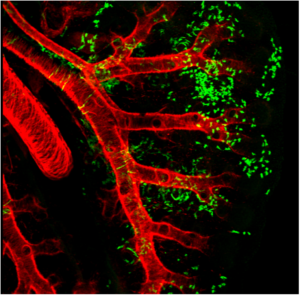
Wnt2+ CPPs (green cells) populate multiple cell lineages in the developing lung including airway and vascular smooth muscle. The smooth muscle of the branching airways and large blood vessels are stained in red.
Adult mesenchymal stem cells (hMSCs)
Adult human mesenchymal stem cells (hMSCs) are the focus of a number of clinical applications. The advantage of hMSCs is that they are immuno-modulatory— capable of modifying or regulating one or more immune functions—and versatile due to the anti-inflammatory and regenerative bioactive molecules they secrete.
hMSCs have the potential to orchestrate reparative processes in diseased or injured tissues. Much of the diversity and uniqueness of hMSCs is defined by their response to the environment of injured tissue. hMSCs are sensitive to their site-specific microenvironment, and scientists anticipate that these cells will deliver the bioactive agents in a site-specific manner quite different from the way pharmaceutical drugs work in the treatment of lung diseases.
hMSCs are non-hematopoietic, multi-potent progenitor cells with the capacity to generate bone marrow stromal cells as well as adipocytes, chondrocytes, and osteocytes in suitable tissue and other organ sites.
Studying lung stem cells sheds light on the causes of lung disease
A better understanding of lung stem cell and progenitor cell biology can improve our knowledge of how the healthy lung works. This in turn will shed light on the causes of lung diseases such as chronic obstructive pulmonary disease (COPD). Such research could lead to the development of new treatments for lung disease. In fact, lung stem cells may be used in future therapies to repair or regenerate the lungs of patients with severe lung damage or disease.
Current research
Lung stem cells have most frequently been identified and characterized in mice. Studies on mice have allowed researchers to identify the differences between embryonic and adult lung stem cells, discover the role of stem cells in lung repair, and investigate how changes to lung stem cells may lead to lung disease. A current focus of research includes testing if the same stem and progenitor cell populations can be identified in human lungs.
repair, and investigate how changes to lung stem cells may lead to lung disease. A current focus of research includes testing if the same stem and progenitor cell populations can be identified in human lungs.
Identifying progenitor and stem cells before and after lung injury
Researchers are also working to determine the role of stem cells in various human lung diseases, including lung cancer and COPD. They have begun examining potential clinical applications of stem cell therapies with several ‘first-in-human’ studies to investigate whether lung stem cells might enhance organ replacement or regeneration in patients.
The future of stem cells in treating lung disease
As researchers continue to improve their understanding of the exact identity and function of human lung stem cells, the potential for clinical applications will be divulged. Researchers will identify methods to control lung stem cells, which can then be tested as treatments for lung diseases. Further research will also investigate the uses of lung stem cells for personalized medicine.
###
- Published in Corporate News / Blog
The History of Research on Adult Stem Cells: We’ve Come a Long Way
An adult stem cell is an undifferentiated cell, found among differentiated cells in tissue or an organ. The adult stem cell can renew itself and can differentiate to yield some or all of the major specialized cell types of the tissue or organ. The primary role of adult stem cells in a living organism is to maintain and repair the tissue in which they are found. Scientists also use the term somatic stem cell to describe adult stem cells, where somatic refers to cells of the body (not the germ cells, sperm or eggs). Unlike embryonic stem cells, which are defined by their origin (cells from the preimplantation-stage embryo), the origin of adult stem cells in some mature tissues is still under investigation.
Research on adult stem cells
Research on adult stem cells has generated a great deal of excitement. Scientists have found adult stem cells in many more tissues than they once thought possible. This finding has led researchers and clinicians to consider whether adult stem cells could be used for transplants. In fact, adult hematopoietic—or blood-forming—stem cells from bone marrow have been used in transplants for more than 40 years. Scientists now have evidence that stem cells exist in the brain and the heart, two locations where adult stem cells were not at first expected to reside. If the differentiation of adult stem cells can be controlled in the laboratory, these cells may become the basis of transplantation-based therapies.
The history of research on adult stem cells began more than 60 years ago. In the 1950s, researchers discovered that the bone marrow contains at least two kinds of stem cells. Hematopoietic stem cells form all the types of blood cells in the body. Bone Marrow stromal stem cells are a multipotent subset of bone marrow stromal cells that are able to form bone, cartilage, stromal cells that support blood formation, fat, and fibrous tissue.
Types of adult stem cells
Bone marrow stem cells are also called mesenchymal stem cells, and were discovered a few years later. These non-hematopoietic stem cells make up a small proportion of the stromal cell population in the bone marrow and can generate bone, cartilage, and fat cells that support the formation of blood and fibrous connective tissue.
In the 1960s, scientists who were studying rats discovered two regions of the brain that contained dividing cells that ultimately become nerve cells, but despite these reports most scientists believed that the adult brain could not generate new nerve cells. It was not until the 1990s that scientists agreed that the adult brain does contain stem cells that are able to generate the brain’s three major cell types—astrocytes and oligodendrocytes, which are non-neuronal cells, and neurons, or nerve cells.
Where are adult stem cells found, and what do they normally do?
Adult stem cells have been identified in many organs and tissues, including brain, bone marrow, peripheral blood, blood vessels, skeletal muscle, skin, teeth, heart, gut, liver, ovarian epithelium, and testis. They are thought to reside in a specific area of each tissue (called a “stem cell niche”). In many tissues, current evidence suggests that some types of stem cells are pericytes, cells that compose the outermost layer of small blood vessels. Stem cells may remain quiescent (non-dividing) for long periods of time until they are activated by a normal need for more cells to maintain tissues, or by disease or tissue injury.
blood vessels, skeletal muscle, skin, teeth, heart, gut, liver, ovarian epithelium, and testis. They are thought to reside in a specific area of each tissue (called a “stem cell niche”). In many tissues, current evidence suggests that some types of stem cells are pericytes, cells that compose the outermost layer of small blood vessels. Stem cells may remain quiescent (non-dividing) for long periods of time until they are activated by a normal need for more cells to maintain tissues, or by disease or tissue injury.
What tests are used to identify adult stem cells?
Scientists often use one or more methods to identify adult stem cells:
• Label the cells in a living tissue with molecular markers and then determine the specialized cell types they generate;
• Remove the cells from a living animal, label them in cell culture, and transplant them back into another animal to determine whether the cells replace (or “repopulate”) their tissue of origin.
Importantly, scientists must demonstrate that a single adult stem cell can generate a line of genetically identical cells that then gives rise to all the appropriate differentiated cell types of the tissue. To confirm experimentally that a putative adult stem cell is indeed a stem cell, scientists show either that the cell can give rise to these genetically identical cells in culture, and/or that a purified population of these candidate stem cells can repopulate or reform the tissue after transplant into an animal.
- Published in Corporate News / Blog
How Clinical Trials on Stem Cell Therapies Work, and Where to Find Them
The most important resource on stem cell clinical trials is registry ClinicalTrials.gov. This registry provides the public with easy access to information on publicly and privately supported clinical studies. The ClinicalTrials.gov web site is maintained by the National Library of Medicine at the National Institutes of Health. Information (NIH) and is provided and updated by the sponsor or principal investigator of the clinical study.
Clinical Trials resources
ClinicalTrials.gov was created as a result of the Food and Drug Administration Modernization Act of 1997 (FDAMA). The site became available to the public in February 2000.
This led to the development of the ClinicalTrials.gov results database, which contains study outcomes.
Another resource, World Health Organization International (WHO) Clinical Trial Registry Platform, contains some additional information not available in the ClinicalTrials.gov registry. However, the difference is relatively small and this registry is easier to work with.
When all stem cell trials are placed on a map, we can see that vast majority of them take place in the U.S., followed by Europe.
Of the 5,101 studies, which were available on the database on August 8, 2015, 1,700 were recruiting patients and 1,769 were completed.
Clinical trials: research in human subjects
The only product that comes up as an approved treatment is Mozobil, a hematopoietic stem cell mobilizer, used in combination with granulocyte-colony stimulating factor, for the treatment of non-Hodgkin lymphoma and multiple myeloma. There are several others, such as Hemacord and Osiris, and Holoclar in Europe. This does not change the fact that approved stem cell treatments are few and far between and that is always worth checking with the relevant local regulator whether the product or treatment intervention is approved in the area or not.
Clinical research in human subjects is conducted in five phases – 0, I, II, III and IV.
• Phase 0 is an exploratory study involving very limited human exposure to the product, with no therapeutic or diagnostic goals.
• Phase 1 studies are usually conducted with healthy volunteers and emphasize safety. The goal is to find out the product’s most frequent and serious adverse events, and explore its biological effects in humans.
• Phase 2 studies gather preliminary data on effectiveness. Safety continues to be evaluated.
• Phase 3 studies gather more information about safety and effectiveness by studying different
populations and different dosages and methods of administration and by using the product in
combination with other drugs or biologics.
• Phase 4 studies occur after the approval of the product for marketing. These studies gather
additional information about a drug’s safety, efficacy, or optimal use. Not all phase 4 trials that
come up are stem cell interventions. Many of them study the effect of approved drugs and
biologics on people who received stem cell therapies.
The vast majority of these studies are in the early stages of development, and there are a considerable group of trials where the phase is not stated.
Clinical Trials: Interventional studies
The majority of stem cell research also consists of interventional studies. This means that human volunteers are assigned to interventions (for example, a medical product or procedure) based on a protocol, and are then evaluated for effects on biomedical or health outcomes.
ClinicalTrials.gov also includes records describing observational studies and programs providing access to  investigational drugs and biologics outside of clinical trials through so called expanded access.
investigational drugs and biologics outside of clinical trials through so called expanded access.
Analysis of sources of funding for stem cell trials shows only a small fraction of stem cell studies funded by the industry. A significant number of trials get funding from the National Institute of Health. All remaining trials get funding from entities categorized as “other,” meaning individuals, universities, and community-based organizations.
The sponsor of a clinical study is the organization or person who oversees the clinical study and is responsible for analyzing the study data. The funder is the organization that provides funding or support for the clinical study. Support may include providing facilities, expertise, or financial resources. Organizations listed as sponsors and collaborators for a study are considered the funders of the study.
There are four types of clinical study funders:
- National Institutes of Health
- Other U.S. Federal agency (for example, the Food and Drug Administration, Centers for Disease Control and Prevention, U.S. Department of Veterans Affairs)
- Industry (pharmaceutical and device companies)
- All others (including individuals, universities, and community-based organizations)
Although the number of studies conducted for a specific condition or disease does not tell us whether the study was successful or not, it does mean that the project was reviewed and approved as viable and that it received funding. The more studies available for a particular disease, the more accumulated knowledge exists, and the more likely it is that continuing research will yield convincing and consistent findings. On the other hand, isolated studies without posted results should be interpreted with caution.
- Published in Corporate News / Blog
What are the potential uses of human stem cells? What obstacles must still be overcome before these potential uses will be realized?
There are many ways in which human stem cells can be used in research and in the clinic. Studies of stem cells continue to yield information about their complex capabilities. A primary goal of this research is to identify how undifferentiated stem cells become the differentiated cells that form the tissues and organs. Scientists know that turning genes on and off is central to this process. Some of the most serious medical conditions, such as cancer and birth defects, are due to abnormal cell division and differentiation.
A more complete understanding of the genetic and molecular triggers of these conditions can yield information about how they arise and suggest new strategies to treat them. Predictably controlling cell proliferation and differentiation requires additional basic research on the molecular and genetic signals that regulate cell division and specialization. While recent developments with induced pluripotent stem cells (iPSCs) suggest some of the specific factors that may be involved, techniques must be developed to introduce these factors safely into the cells and control the processes that are induced by these factors.
Human stem cells and drug testing
Human stem cells are also being used to test new drugs. New medications are tested for safety on differentiated cells generated from human pluripotent cell lines. Other kinds of cell lines have a long history of being used in this way. Cancer cell lines, for example, are used to screen potential anti-tumor drugs. The availability of pluripotent stem cells would allow drug testing on a wider range of cell types. However, to screen drugs effectively, the conditions must be identical when comparing different drugs. Therefore, scientists must be able to precisely control the differentiation of stem cells into the specific cell type on which drugs will be tested.
For some cell types and tissues, current knowledge of the signals controlling differentiation falls short of being able to mimic these conditions precisely to generate pure populations of differentiated cells for each drug being tested.
Human stem cells, cell and tissue generation
Perhaps the most important potential application of human stem cells is the generation of cells and tissues that could be used for cell-based therapies. Today, donated organs and tissues are often used to replace ailing or destroyed tissue, but the need for transplantable tissues and organs far outweighs the available supply. Stem cells, directed to differentiate into specific cell types, offer the possibility of a renewable source of replacement cells and tissues to treat diseases including macular degeneration, spinal cord injury, stroke, burns, heart disease, diabetes, osteoarthritis, and rheumatoid arthritis.
used for cell-based therapies. Today, donated organs and tissues are often used to replace ailing or destroyed tissue, but the need for transplantable tissues and organs far outweighs the available supply. Stem cells, directed to differentiate into specific cell types, offer the possibility of a renewable source of replacement cells and tissues to treat diseases including macular degeneration, spinal cord injury, stroke, burns, heart disease, diabetes, osteoarthritis, and rheumatoid arthritis.
- Published in Corporate News / Blog
Mending a Broken Heart and Addressing Diabetes with Adult Stem Cells
Researchers are learning about mending a broken heart–that is, how to generate healthy heart muscle stem cells in the laboratory and then transplant those cells into patients with chronic heart disease. Preliminary research in mice and other animals indicates that bone marrow stromal cells, transplanted into a damaged heart, can have beneficial effects. Whether these cells can generate heart muscle cells or stimulate the growth of new blood vessels that repopulate the heart tissue, or help through some other mechanism is actively under investigation.
Stem Cell Research: Mending a Broken Heart
For example, injected cells may accomplish repair by secreting growth factors, rather than actually incorporating into the heart. Promising results from animal studies have served as the basis for a small number of exploratory studies in humans. Other recent studies in cell culture systems indicate that it may be possible to direct the differentiation of adult bone marrow cells into heart muscle cells.
Can Stem Cells Mend a Broken Heart? For that matter, what can stem cells do to treat all cardiovascular diseases including hypertension, coronary heart disease, stroke, and congestive heart failure? Cardiovascular disease (CVD) has ranked the number one cause of death in the United States every year since 1900 except 1918, when the nation struggled with an influenza epidemic.
Nearly 2,600 Americans die of CVD each day—roughly one person every 34 seconds. Given the country’s large aging population and the relatively dramatic recent increases in the prevalence of cardiovascular risk factors such as obesity and type 2 diabetes, CVD will be a significant health concern for decades to come.
Strategies to treat heart disease with stem cells
Cardiovascular disease can deprive heart tissue of oxygen, thereby killing cardiac muscle cells (cardiomyocytes). This loss triggers a cascade of detrimental events, including formation of scar tissue, an overload of blood flow and pressure capacity, the overstretching of viable cardiac cells attempting to sustain cardiac output leading to heart failure, and eventual death. Restoring damaged heart muscle tissue, through repair or regeneration, is therefore a potentially new strategy to treat heart failure.
The use of adult-derived stem cells for cardiac repair is an active area of research. A number of stem cell types, including cardiac stem cells that naturally reside within the heart, myoblasts (muscle stem cells), adult bone marrow-derived cells including mesenchymal cells (bone marrow-derived cells that give rise to tissues such as muscle, bone, tendons, ligaments, and adipose tissue), endothelial progenitor cells (cells that give rise to the endothelium, the interior lining of blood vessels), and umbilical cord blood cells, have been investigated as possible sources for regenerating damaged heart tissue. All have been explored in mouse or rat models, and some have been tested in larger animal models, such as pigs.
A few small studies have also been carried out in humans, usually in patients who are undergoing open-heart surgery. Several of these have demonstrated that stem cells that are injected into the circulation or directly into the injured heart tissue appear to improve cardiac function and/or induce the formation of new capillaries. The mechanism for this repair remains controversial, and the stem cells likely regenerate heart tissue through several pathways. However, the stem cell populations that have been tested in these experiments vary widely, as do the conditions of their purification and application. Although much more research is needed to assess the safety and improve the efficacy of this approach, these preliminary clinical experiments show how stem cells may one day be used to repair damaged heart tissue, thereby reducing the burden of cardiovascular disease.
Treating type I diabetes
In people who suffer from type 1 diabetes, the cells of the pancreas that normally produce insulin are destroyed by the patient’s own immune system. New studies indicate that it may be possible to direct the differentiation of human embryonic stem cells in cell culture to form insulin-producing cells that eventually could be used in transplantation therapy for persons with diabetes.
To realize the promise of novel cell-based therapies for such pervasive and debilitating diseases, scientists must be able to manipulate stem cells so that they possess the necessary characteristics for successful differentiation, transplantation, and engraftment. The following is a list of steps in successful cell-based treatments that scientists will have to learn to control to bring such treatments to the clinic. To be useful for transplant purposes, stem cells must be reproducibly made to:
- Reproduce extensively and generate sufficient quantities of cells for making tissue.
- Differentiate into the desired cell type(s).
- Survive in the recipient after transplant.
- Integrate into the surrounding tissue after transplant.
- Function appropriately for the duration of the recipient’s life.
- Avoid harming the recipient in any way.
Also, to avoid the problem of immune rejection, scientists are achieving good results with strategies that use the patient’s own stem cells to generate tissue that will not be rejected.
- Published in Corporate News / Blog
Stem Cell Research and Stem Cell Therapy: When can stem cells be used to treat patients?
The difference between stem cell research and therapy is in the scientific evidence that supports therapeutic intervention to be beneficial for the patient.
Stem cells have the remarkable potential to develop into many different types of cells in the body during early life and growth. In addition, in many tissues, stem cells serve as a sort of internal repair system, dividing essentially without limit to replenish other cells as long as the individual is alive. When a stem cell divides, each new cell has the potential either to remain a stem cell or become another type of cell with a more specialized function, such as a muscle cell, a red blood cell, or a brain cell.
Stem cell research on adult stem cells
Stem cells are distinguished from other cell types by two important characteristics. First, they are unspecialized cells capable of renewing themselves through cell division, sometimes after long periods of inactivity. Second, under certain physiologic or experimental conditions, they can be induced to become tissue- or organ-specific cells with special functions. In some organs, such as the gut and bone marrow, stem cells regularly divide to repair and replace worn out or damaged tissues. In other organs, such as the pancreas and the heart, stem cells only divide under special conditions.
Until recently, scientists primarily worked with two kinds of stem cells from animals and humans: embryonic stem cells and non-embryonic “somatic” or “adult” stem cells in stem cell research.
In 2006, researchers made a breakthrough by identifying conditions that would allow some specialized adult cells to be “reprogrammed” genetically to assume a stem cell-like state. This new type of stem cell is called induced pluripotent stem cells (iPSCs).
Stem cells are important for living organisms for many reasons. In the 3- to 5-day-old embryo, called a blastocyst, the inner cells give rise to the entire body of the organism, including all of the many specialized cell types and organs such as the heart, lungs, skin, sperm, eggs and other tissues. In some adult tissues, such as bone marrow, muscle, and brain, discrete populations of adult stem cells generate replacements for cells that are lost through normal wear and tear, injury, or disease.
Stem cell research for treating disease
Given their unique regenerative abilities, stem cells offer new potentials for treating diseases such as diabetes, and heart disease. However, much work remains to be done in the laboratory and the clinic to understand how to use these cells for cell-based therapies to treat disease, which is also referred to as regenerative or reparative medicine.
Laboratory studies of stem cells enable scientists to learn about the cells’ essential properties and what makes them different from specialized cell types. Scientists are already using stem cells in the laboratory to screen new drugs and to develop model systems to study normal growth and identify the causes of birth defects.
Research on stem cells continues to advance knowledge about how an organism develops from a single cell and how healthy cells replace damaged cells in adult organisms. Stem cell research is one of the most fascinating areas of contemporary biology, but, as with many expanding fields of scientific inquiry, research on stem cells raises scientific questions as rapidly as it generates new discoveries.
In 1964, the World Medical Association developed the Declaration of Helsinki as a statement of
ethical principles for medical research involving human subjects. It includes research on identifiable human material and data, last amended in October 2013.
According to the Helsinki Declaration, in the treatment of an individual patient where proven interventions do not exist or other known interventions have been ineffective, the physician, after seeking expert advice, with informed consent from the patient or a legally authorized representative, may use an unproven intervention if in the physician’s judgement it offers hope of saving life, re-establishing health or alleviating suffering.
Intervention should subsequently be made the object of research, designed to evaluate its safety and efficacy. In all cases, new information must be recorded and, where appropriate, made publicly available.
- Published in Corporate News / Blog
Stem Cell Myths, Busted
The term stem cell research gleans different reactions from people, both in the medical community and the wider public. Still an emerging science, stem cell research is shrouded by many myths and misconceptions. Here, we take on some of the most predominant myths to discuss the misconceptions and clarify the facts regarding this fast-growing branch of medicine.
Stem cell myths
Myth #1: Stem cells only come from embryos.
FACT: False. Stem cells exist in all bodies, from embryos to adults.
Embryonic stem cells come from the early embryo, and have the potential to produce all the specialized cells of the body. Because of this, they hold great promise for studying and potentially treating disease and injuries. Tissue or “adult” stem cells are found in the body throughout our lives. These cells maintain and repair many tissues in the body. Examples of these cells include blood stem cells, muscle stem cells, bone marrow stem cells, adipose tissue (fat) stem cells and skin stem cells. Some of these adult stem cells are used in established medical and aesthetic treatments.
Myth #2: Induced pluripotent stem cells (iPSCs) eliminate the need for embryonic cells
FACT: False. Research is needed on all types of cells because it is not clear which cells will be most useful for which types of application. For the foreseeable future, side-by-side research on both embryonic and induced pluripotent stem cells is needed. Global Stem Cell Group’s research and treatment products use no embryonic stem cells.
Myth #3: Stem cell research leads to cloning humans.
FACT: False. Most countries prohibit this type of cloning.
In most countries, even attempting to clone a human being is illegal. Some countries do allow something called “therapeutic cloning” for the purposes of studying a disease. In this procedure, scientists isolate embryonic stem cells from a cloned blastocyst (early stage embryo) but do not transfer the blastocyst into a womb. In therapeutic cloning, the blastocyst is not transferred to a womb. Instead, embryonic stem cells are isolated from the cloned blastocyst. These stem cells are genetically matched to the donor organism for studying genetic disease. For example, stem cells could be generated using the nuclear transfer process described above, with the donor adult cell coming from a patient with diabetes or Alzheimer’s. The stem cells could be studied in the laboratory to help researchers understand what goes wrong in diseases like these.
Therapeutic cloning also could be used to generate cells that are genetically identical to a patient’s. A patient transplanted with these cells would not suffer the problems associated with transplant rejection. To date, no human embryonic stem cell lines have been derived using therapeutic cloning.
Myth #4: Adult stem cells are only found in adults
FACT: False. There are three different types of stem cells: embryonic stem cells, induced pluripotent stem cells and tissue specific stem cells. It’s the tissue stem cells that are often called “adult” stem cells, but these “adult” stem cells are found in people of all ages. (See myth #1).
Stem cell myths: research
Myth #5: Embryonic stem cell research is banned in Europe.
FACT: False. The laws vary across the EU.
EU member states have diverging regulatory positions on human embryonic stem cell research. For instance, in Germany, the use of embryos for research is heavily restricted under the Embryo Protection Act (Embryonenschutzgesetz) of 1991, which makes the derivation
of embryonic stem cell lines a criminal offense. But in the UK, embryonic stem cell research is allowed, subject to licensing from the Human Fertilization and Embryology Authority (HFEA). Click here for country by country overviews for more details. Under the previous two European Framework programs (FP6 and F7), as well as the current program, Horizon 2020, human embryonic stem cell research can be funded, provided that the work is permitted by law in the country where it is to take place.
Myth #6: Stem cell research and treatment is against the law in the US.
FACT: False. The FDA does not regulate the practice of medicine, but rather drugs and medical devices and which of these can be marketed in the US. Under federal law, cultured (grown) stem cell products are considered a drug, but are not illegal. Adult stem cells, however, are not cultured—they exist in our bodies throughout our organs, blood, skin, teeth, fat, bone marrow and other places.
Adult stem cell therapy is currently used in the United States to treat conditions such as leukemia and other illnesses. Bone marrow consists of stem cells which have been transplanted for years in the US.
Global Stem Cells Group offers stem cell treatments in countries where stem cell therapy is approved and regulated with no appreciable difference in safety record.. Stem cell therapy technology is still under review by the FDA.
Stem cell myths: therapies
Myth #7: Bone marrow is the best source of stem cells.
FACT: False. Bone marrow is just one source of stem cells. Bone marrow stem cells have been studied for decades, and have been used to treat certain types of cancer. A great deal of research has been dedicated to understanding this source of stem cells and their potential. Bone marrow contains a number of different kinds of stem cells, one of which is mesenchymal stem cells. However, mesanchymal stem cells can also be found in adipose (fat) tissue at nearly 2000 times the frequency of bone marrow.
Mesenchymal cells have the capability to become different types of tissues (blood vessels, muscle tissue, etc.) and are capable of communicating with other cells. In combination with other proteins, molecules and regenerative cells found in adipose tissue, they also have the ability to reduce inflammation, regenerate damaged tissue, and grow new blood vessels, a process known as angiogenesis. Stem cells from adipose tissue are more accessible and abundant. They can be processed immediately and reintroduced into the body right away.
Myth #8: There is a risk of rejection with stem cell therapy.
FACT: False. When a patient’s stem cells are derived from his or her own body (such as fat tissue), there is no risk of rejection. In fact, studies thus far have indicated no safety issues with fat-derived autologous (from self) stem cells. Since these stem cells come from your own body, the risk of rejection is eliminated.
###
- Published in Corporate News / Blog
Global Stem Cells Group Launches Two Stem Cell Treatment Centers in Arica and Iquique, Chile

Global Stem Cells Group announces the launch of two new stem cell treatment clinics in the cities of Arica and Iquique in northern Chile. The facilities are part of the international biotech company’s expanding presence in Latin America.
MIAMI, May 31, 2016—Global Stem Cells Group, a leading international biotechnology company, announces the launch of operations at two new GSCG clinics in the cities of Arica and Iquique in northern Chile. The facilities are part of GSCG’s expanding presence in Latin America.
Both the Arica and Iquique clinics offer the most advanced protocols and techniques in stem cell medicine to patients from around the world.

The clinics are headed by stem cell specialists Victor Perez, M.D., and Duval Aguirre, M.D., and will offer treatments in chronic degenerative conditions, Type 2 diabetes, COPD, traumatology and sports medicine.
Global Stem Cells Group, has been expanding its clinical presence throughout Latin America and worldwide by partnering with qualified physicians experienced in stem cell therapies to open new clinics. The new Arica and Iquique clinics are certified for the medical tourism market.
Global Stem Cells Group is committed to the highest standards in service and technology, expert and compassionate care, and a philosophy of exceeding the expectations of their international patients.
For more information, visit the Global Stem Cells Group website, Email bnovas@stemcellsgroup.com, or call 305-560-5337.
About the Global Stem Cells Group:

Global Stem Cells Group, Inc. is the parent company of six wholly owned operating companies dedicated entirely to stem cell research, training, products and solutions. Founded in 2012, the company combines dedicated researchers, physician and patient educators and solution providers with the shared goal of meeting the growing worldwide need for leading edge stem cell treatments and solutions.
With a singular focus on this exciting new area of medical research, Global Stem Cells Group and its subsidiaries are uniquely positioned to become global leaders in cellular medicine.
Global Stem Cells Groups corporate mission is to make the promise of stem cell medicine a reality for patients around the world. With each of GSCGs six operating companies focused on a separate research-based mission, the result is a global network of state-of-the-art stem cell treatments.
To view this press release live online, click here
###
- Published in Press Releases