Global Stem Cells Group Announces Stem Cell Training Course Scheduled in Barcelona
Global Stem Cells Group and its subsidiary Stem Cell Training, Inc., have announced plans to hold a stem cell training course in Barcelona, Spain, Nov. 11 – 12, 2016. Orthopedic and cosmetic surgeon J. Victor Garcia Gimenez, M.D. will conduct the course for qualified physicians and medical professionals.
[su_spacer]

J. Victor Garcia Gimenez, M.D.
Garcia Gimenez, a member of the Global Stem Cells Group Advisory Board, first conducted the course for GSCG in 2014. The
course is part of the Miami-based biotech company’s growth in the European market.
The training course was developed for physicians and high-level practitioners to learn clinical protocols and state-of-the-art techniques for isolating and re-integrating adipose- and bone marrow-derived stem cells. Stem cells are harvested from the patient’s own body and redistributed to areas of the body receiving treatment. Patients experience an effective, non-invasive procedure, and a faster recovery period with little to no downtime.
Garcia Gimenez is a specialist in orthopedic and cosmetic surgery, president of Therapeutic Confrontations (CONFTERA), and practices cosmetic and anti-aging medicine, as well as aesthetic therapies in Barcelona. He is the president of the Spanish Society of Medicine and Cosmetic Surgery; co-director of the UAB-SEMCC; Chairman for Spain of the International Academy of Cosmetic Surgery, in addition to other medical and professional boards.
The stem cell training course will be offered through Global Stem Cells Group affiliate Stem Cell Training, Inc.
To learn more, visit the Global Stem Cells Group website, or the Stem Cell Training website, email bnovas(at)regenestem(dot)com, or call +1 305 560 5337.
About Global Stem Cell Group:
Global Stem Cells Group, Inc. is the parent company of six wholly owned operating companies dedicated entirely to stem cell research, training, products and solutions. Founded in 2012, the company combines dedicated researchers, physician and patient educators and solution providers with the shared goal of meeting the growing worldwide need for leading edge stem cell treatments and solutions. With a singular focus on this exciting new area of medical research, Global Stem Cells Group and its subsidiaries are uniquely positioned to become global leaders in cellular medicine.
About Stem Cell Training, Inc.:
 Stem Cell Training, Inc. is a multi-disciplinary company offering coursework and training in 35 cities worldwide. The coursework offered focuses on minimally invasive techniques for harvesting stem cells from adipose tissue, bone marrow and platelet-rich plasma. By equipping physicians with these techniques, the goal is to enable them to return to their practices, better able to apply these techniques in patient treatment.
Stem Cell Training, Inc. is a multi-disciplinary company offering coursework and training in 35 cities worldwide. The coursework offered focuses on minimally invasive techniques for harvesting stem cells from adipose tissue, bone marrow and platelet-rich plasma. By equipping physicians with these techniques, the goal is to enable them to return to their practices, better able to apply these techniques in patient treatment.
To view this press release live online, click here.
###
- Published in Press Releases
Texas Man Becomes First Adult in the U.S. to Receive Updated Stem Cell Transplant to Treat Leukemia
Chuck Dandridge, a Mansfield, Texas resident, became the first adult in the U.S. to receive a newly modified stem cell transplant that uses genetically engineered blood cells from a family member. The milestone was announced by researchers at UT Southwestern Medical Center’s Harold C. Simmons Comprehensive Cancer Center in Dallas, where the procedure was performed.
Dandridge’s medical journey began in 2013, with a routine doctor’s visit to check his cholesterol levels; lab tests revealed low blood counts and further testing confirmed Dandridge’s diagnosis of myelodysplastic syndrome, also called pre-leukemia or MDS. By 2014, the leukemia had progressed to acute myeloid leukemia (AML), which, according to the National Cancer Institute, affects more than 20,000 Americans annually.
Dandridge was referred to UT Southwestern’s Simmons Cancer Center, where his leukemia was tested for genetic mutations.
“We wanted to know whether he had specific mutations in his cancer cells,” says

Jon Dandridge, Madhuri Vusirikala, M.D., and Chuck Dandridge at the Simmons Cancer Center. Photo: UT Southwestern Medical Center.
“We found a mutation called IDH 2, which causes the body to produce an abnormal protein that promotes excessive cell growth. If you can target that mutation and stop the abnormal protein from being produced, then cells start behaving normally.”
Dandridge enrolled in a UT Southwestern clinical trial for a therapy called AG-221. He took four pills each morning for the next eight months. During that time, Dandridge saw marked improvement although he did not go into complete remission, according to Vusirikala.
That success made him eligible for a potentially curative stem cell transplant. But finding a donor proved challenging.
“The best chance of finding a full match is usually a full sibling; however, Chuck has no full siblings,” Vusirikala says. Additionally, Dandridge is African American, and minorities are under-represented in the National Marrow Donor Registry—about 70 percent of registry donors are Caucasian. The search for an unrelated donor was unsuccessful.
Vusirikala says that he knew Dandridge’s daughter and his son would be at least a half match. Since using a same-sex donor is preferred, as it reduces the risk of complications, his son Jon, 31, emerged as the best choice. But the risk of graft-versus-host-disease (GvHD) following a transplant using a half-match is very high, so they needed a better way to deal with the GvHD risk.
Once again, Mr. Dandridge volunteered for a cutting-edge clinical trial, known as BP-001, which processed the stem cells used in the transplant to reduce the risk of rejection and engineered blood cells that can be targeted if GvHD develops after the transplant.
 The processes being tested in BP-001 are in clinical development by Houston-based Bellicum Pharmaceuticals. The study is evaluating patients with blood cell cancers who have a peripheral blood stem cell transplant from a partially matched relative. Immune cells (T cells) from the related donor are separated from the rest of the stem cells and genetically engineered in the Bellicum laboratory, and then given to the patient along with the stem cell transplant.
The processes being tested in BP-001 are in clinical development by Houston-based Bellicum Pharmaceuticals. The study is evaluating patients with blood cell cancers who have a peripheral blood stem cell transplant from a partially matched relative. Immune cells (T cells) from the related donor are separated from the rest of the stem cells and genetically engineered in the Bellicum laboratory, and then given to the patient along with the stem cell transplant.These engineered T cells are modified to include a suicide gene with the help of a retrovirus. If the patient develops GvHD after transplant, the side-effect can be treated by giving a drug called rimiducid to activate the suicide gene and cause the activated GvHD-causing cells to be eliminated. The stem cells given for the transplant were also processed prior to giving them back to Dandridge to reduce the risk of graft rejection as well as GvHD.
The genetically engineered blood cells were transplanted from Dandrige’s son, Jon, 31, to the father in three, two-hour infusions at William P. Clements Jr. University Hospital in July, 2015, and today the elder Mr. Dandridge’s leukemia is in remission. His immune system is recovering, and the former Norman, Oklahoma YMCA CEO is now mentoring first-time CEOs for the YMCA.
###
- Published in Corporate News / Blog
Insulin-producing Stem Cells Grown in the Lab Mark a New Era in Stem Cell Therapies for Diabetes
A new discovery by researchers on how to activate lab-grown beta cells to mature into functioning cells that produce and release insulin in response to glucose take a significant step toward a cell therapy treatment for diabetes.
Difficulties in manipulating beta cells derived from human stem cells to mature beyond the precursor stage into fully functioning insulin releasers has been an on-going challenge for researchers..
However, researchers from the Salk Institute for Biological Studies and a team of researchers have achieved this goal with lab-grown beta cells by activating a protein called estrogen-related receptor γ (ERRγ). Their study findings were recently published in the journal Cell Metabolism.
Self-renewing capacity of human pluripotent stem cells (hPSCs)
Ronald Evans, senior author of the study, titled, “ERRγ Is required for the Metabolic Maturation of Therapeutically Functional Glucose-Responsive β Cells,”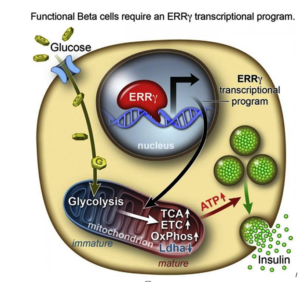 says the self-renewing capacity of human pluripotent stem cells (hPSCs) and their ability to differentiate into most cell types—from neurons to skin cells, to muscles cells and insulin-producing pancreatic beta cells—has inspired many research teams to find ways to make glucose-responsive beta cells in the lab.
says the self-renewing capacity of human pluripotent stem cells (hPSCs) and their ability to differentiate into most cell types—from neurons to skin cells, to muscles cells and insulin-producing pancreatic beta cells—has inspired many research teams to find ways to make glucose-responsive beta cells in the lab.
Evans and his research team discovered the answer to the insulin-releasing cell conundrum, and summed it up thusly:
“In a dish, with this one switch, it’s possible to produce a functional human beta cell that’s responding almost as well as the natural thing.”
Evans, a molecular biologist at the Salk Institute, says that to create the different types of cells in the lab, researchers coax the pluripotent stem cells (hPSCs) down the various branching paths that fetal cells normally travel in order to differentiate into the various cell types. However, he explains there are many developmental points in this process, and in the case of lab-grown pancreatic beta cells, research kept getting stuck at an early stage.
Adult beta cells have more ERRγ protein for a very energy-intensive process
In order to determine what might trigger the next step in getting the cells to mature, the researchers compared transcriptomes of adult and fetal beta cells. The transcriptome contains, among other things, the full catalog of molecules that switch genes on and off in the genome, which led them to discover that the nuclear receptor protein ERRγ was more abundant in adult beta cells. The team was already familiar with the protein’s role in muscle cells and had studied its ability to enhance endurance running.
Evans says that in muscles, protein promotes greater growth of mitochondria—the power generators inside cells that accelerate the burning of sugars and fats to make energy.
“It was a little bit of a surprise to see that beta cells produce a high level of this regulator,” Evans says. “But beta cells have to release massive amounts of insulin quickly to control sugar levels. It’s a very energy-intensive process.”
The research team then decided to run some tests to look more closely at what role ERRγ might play in insulin-producing beta cells.
A new era in creating functional, insulin-producing beta cells
 After they genetically engineering a deficiency of ERRy in mice, the researchers found the animals’ beta cells did not produce insulin in response to spikes in blood sugar.
After they genetically engineering a deficiency of ERRy in mice, the researchers found the animals’ beta cells did not produce insulin in response to spikes in blood sugar.
Next they tried to get beta cells made from hPSCs to produce more ERRγ, and it worked! The cells in culture began to respond to glucose and release insulin.
Finally, the team transplanted the lab-grown insulin-producing beta cells into diabetic mice and found that from day one, the cells produced insulin in response to glucose spikes in the animals’ blood.
Evans and the research team were justifiably excited by the results. It appears that just switching on the ERRγ protein is sufficient to get the lab-grown beta cells to mature and produce insulin in response to glucose – both in cultures and in live animals.
Speculating on the implications of their findings, Evans suggests that when a fetus is developing, because it gets a steady supply of glucose from the mother, it does not need to produce insulin to regulate its blood sugar, so the switch is inactive. But, when the baby is born and takes its first breath and takes in oxygen, this activates the switch.
Previous lab attempts to produce beta cells got stuck at the fetal stage. The Salk Institute researchers discovered how to take it to the adult stage, using the same protein that is switched on in nature.
“I believe this work transitions us to a new era in creating functional beta cells at will,” Evans says.
He and his research team now plan to examine how the switch might work in more complex models of diabetes treatments.
The Salk Institute study proceeds another study Medical News Today in which researchers generated mini-stomachs that produce insulin when transplanted into mice.
###
- Published in Corporate News / Blog
Amazing Stem Cell Research Breakthroughs You Never Heard of
Scientists have been studying stem cells for decades, and many of their findings, all pretty remarkable, aren’t widely circulated. Periodically, we will share one of these stem cell research breakthroughs here on this blog.
Summary: The skin renews, heals wounds, and regenerates the hair that covers it thanks to a small group of stem cells. These cells continually produce new ones, which appear on the skin surface after a few days. A 2008, released online July 28, 2016, has identified two proteins that are fundamental to conserve skin stem cells, and shows that without these proteins these cells are lost. Researchers find that these proteins, Dnmt3a and Dnmt3b, are altered similarly to tumor cells found in leukemia, lung cancer and colon cancer, which may help researchers discover if the proteins contribute to tumor development.
Amazing stem cell breakthroughs
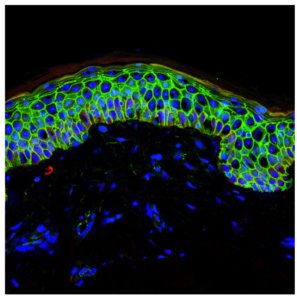
Section of the epidermis showing all its layers, with cell borders in green and cell nuclei in blue (photo: Melissa Mangione)
Researchers identify two proteins— Dnmt3a and Dnmt3b—fundamental to conserving skin stem cells.
The study examines the continuous regeneration of the skin and hair that covers it, thanks to a small group of stem cells. Study researchers identified two proteins— Dnmt3a and Dnmt3b—that are fundamental to conserving skin stem cells. “Without these proteins, skin stem cells are not activated and the stem cells collapse and disappear from the tissue,” according Benitah, head of the Stem Cells and Cancer lab at IRB Barcelona.
Lorenzo Rinaldi, a la Caixa PhD student and first author of the study, identified all the regions of the genome that harbors these proteins. Rinaldi has observed that these two proteins exert their activity on gene enhancers and super-enhancers. Researchers were surprised to see that the two proteins, which had previously been associated with gene repression through DNA methylation, are activated in the most transcriptionally active regions of stem cells.
Researchers observe Dnmt3a and Dnmt3b at the genomic level for the first time
“We had never observed this activity because we were unable to study the global distribution
 of Dnmt3a and Dnmt3b at the genomic level,” Rinaldi says. “Thanks to advances in sequencing techniques, more researchers are observing the very mechanism that we have described.”
of Dnmt3a and Dnmt3b at the genomic level,” Rinaldi says. “Thanks to advances in sequencing techniques, more researchers are observing the very mechanism that we have described.”Of the 12,000 gene enhancers in the genome, about 300 are super-enhancers related to stem cells. The two proteins exert their function in these regions in order to trigger the approx. 1,000 genes required for the self-renewing capacity of skin stem cells. By methylating the super-enhancer, these proteins trigger the first step of the machinery that leads to the amplified expression of these essential genes for the stem cell.
Link to cancer
Among the various features related to tumor cells are three components:
• these cells show altered DNA methylation.
• gene enhancers, in addition to the bodies of the genes themselves, are highly mutated. These observations have been made possible thanks to mass sequencing of tumor cell genomes.
• these two proteins, Dnmt3a and Dnmt3b, are altered in many types of tumors, such as those encountered in leukemia, lung cancer and colon cancer.
Each of these three components is associated with the development of various kinds of cancer. Given that these proteins activate gene expression enhancers through DNA methylation, researchers believe that further studies of them in cancer cells would be helpful in determining whether they participate in tumor development.
The study was funded by the Spanish Ministry of Economy and Competitiveness and ERDFs. Benitah’s lab is also supported by The European Council for Research (ERC), the Worldwide Cancer Research Foundation, the Fundació Marató de TV3, the Fundación Vencer el Cáncer, the Fundación Botín and the Government of Catalonia.
###
- Published in Corporate News / Blog
Global Stem Cells Group Announces Training Course Scheduled in Manila, Philippines
Global Stem Cells Group has scheduled the first stem cell training course to be held in the Philippines Oct. 14-15, 2016. The course will be available to physicians from the Philippines, Thailand and Singapore who are qualified for training in the latest stem cell therapies.
MIAMI, Aug. 2, 2016—Global Stem Cells Group, a world leader in regenerative medicine, has announced the first stem cell training course to be held in Manila,

Makata, Manilla, Philippines
Philippines has been scheduled for Oct. 14 – 15, 2016. The course is part of a collaborative agreement between GSCG and Manila-based Eric Yalung, M.D., to train qualified physicians from the Philippines, Thailand and Singapore in the latest adipose and bone marrow therapies. The announcement signals GSCG’s renewed focus on the South East Asia markets, including permanent stem cell training centers in the Philippines and South Korea.
Adipose and Bone Marrow Stem Cell Training Course
The two-day intensive “Adipose and Bone Marrow Training Course” program will be held for referred physicians. In addition, Global Stem Cells Group will host training for its graduate course, “Diplomat in Stem Cell Training and Tissue Engineering,” with dates to be announced.
According to Global Stem Cells Group CEO Benito Novas, the agreement is the latest in the international biotech company’s ongoing expansion efforts to bring stem cell treatments to communities worldwide.
For more information, visit the Global Stem Cells Group website, email bnovas(at)stemcellsgroup (dot)com, or call (305) 560-5337.
About Global Stem Cell Group:
Global Stem Cells Group is the parent company of six wholly owned operating companies dedicated entirely to stem cell research, training, products and solutions. Founded in 2012, the company combines dedicated researchers, physician and patient educators and solution providers with the shared goal of meeting the growing worldwide need for leading edge stem cell treatments and solutions. With a singular focus on this exciting new area of medical research, Global Stem Cells Group and its subsidiaries are uniquely positioned to become global leaders in cellular medicine.
is the parent company of six wholly owned operating companies dedicated entirely to stem cell research, training, products and solutions. Founded in 2012, the company combines dedicated researchers, physician and patient educators and solution providers with the shared goal of meeting the growing worldwide need for leading edge stem cell treatments and solutions. With a singular focus on this exciting new area of medical research, Global Stem Cells Group and its subsidiaries are uniquely positioned to become global leaders in cellular medicine.
Global Stem Cells Group’s corporate mission is to make the promise of stem cell medicine a reality for patients around the world. With each of GSCG’s six operating companies focused on a separate research-based mission, the result is a global network of state-of-the-art stem cell treatments.
About Stem Cells Training:
Stem Cell Training, Inc. is a multi-disciplinary company offering coursework and training in 35 cities worldwide. Coursework  offered focuses on minimally invasive techniques for harvesting stem cells from adipose tissue, bone marrow and platelet-rich plasma. By equipping physicians with these techniques, the goal is to enable them to return to their practices, better able to apply these techniques in patient treatments.
offered focuses on minimally invasive techniques for harvesting stem cells from adipose tissue, bone marrow and platelet-rich plasma. By equipping physicians with these techniques, the goal is to enable them to return to their practices, better able to apply these techniques in patient treatments.
The company’s training courses are designed to make the best use of stem cell technology available to treat various diseases in a manner that is accessible to everyone. Stem Cell Training, Inc.’s mission is to introduce the promising world of cellular medicine to everyone who can benefit from its application, and to provide high quality, effective and efficient training that complies with the highest medical standards to physicians worldwide.
To view this press release live online, click here
###
- Published in Press Releases
Global Stem Cells Group Subsidiary Adimarket Announces Progenikine™ SVF Closed System Now Available to Purchase Online
Global Stem Cells Group subsidiary Adimarket announces that Progenikine™, is now available to purchase through the Adimarket website. Progenikine is the new SVF closed system kit utilizing EmCyte technology and containing all the elements necessary to process adipose tissue and obtain stromal vascular fraction in a sterile environment for stem cell therapies.
MIAMI, July 30, 2016–Adimarket, a subsidiary of Global Stem Cells Group, Inc., has announced that Progenikine™, the new and approved SVF closed system kit using EmCyte technology, is now available to purchase online through the Adimarket website. The Progenikine kit contains all the elements necessary to process adipose tissue and obtain stromal vascular fraction (SVF) in a closed environment.
A growing number of physicians are switching to the Progenikine kit system, as it provides the perfect preparation for virtually all clinical applications.
Built with EmCyte Technology, the kit has been independently reviewed and proven in various critical performance points that make a difference in patient outcomes. The Progenikine system allows entire procedure s to be performed in a sterile closed system. Currently, the Progenikine kit is being used in topical procedures such as intra-articular injection for osteoarthritis of the knee and hip, cosmetic surgery and acne scarring, dermal injection, stem cell enriched fat transfer, wounds, chronic ulcers, and other chronic conditions.
s to be performed in a sterile closed system. Currently, the Progenikine kit is being used in topical procedures such as intra-articular injection for osteoarthritis of the knee and hip, cosmetic surgery and acne scarring, dermal injection, stem cell enriched fat transfer, wounds, chronic ulcers, and other chronic conditions.
Adipose derived stem cells (ASCs) are used by physicians for a variety of indications. Most commonly, ASCs are isolated at the point of care from lipoaspirate (derived from liposuction) tissue as the stromal vascular fraction (SVF), harvested from the patient and immediately administered to the patient as an injection, or used to enrich fat grafts. Isolation of ASCs from adipose tissue is a relatively simple process performed routinely in cell biology laboratories, but isolation at the point of care for immediate clinical administration requires special methodology to prevent contamination, ensure integrity of the clinical procedure, and comply with regulatory requirements.
Developed  in conjunction with Patrick Pennie, EmCyte CEO, Progenikine fuses elements from EmCyte systems with the Global Stem Cells Group SVF protocols. The kit can provide a low cost, rapid and simple alternative to traditional methods of isolating ASCs, particularly when smaller quantities are needed.
in conjunction with Patrick Pennie, EmCyte CEO, Progenikine fuses elements from EmCyte systems with the Global Stem Cells Group SVF protocols. The kit can provide a low cost, rapid and simple alternative to traditional methods of isolating ASCs, particularly when smaller quantities are needed.
To learn more about the Progenikine kit, visit the Adimarket website, email bnovas(at)stemcellsgroup(dot)com, or call 305-560-5337.
About Global Stem Cells Group:
Global Stem Cells Group is the parent company of six wholly owned operating companies dedicated entirely to stem cell research, training, products and solutions. Founded in 2012, the company combines dedicated researchers, physician and patient educators and solution providers with the shared goal of meeting the growing worldwide need for leading edge stem cell treatments and solutions.
With a singular focus on this exciting new area of medical research, Global Stem Cells Group and its subsidiaries are uniquely positioned to become global leaders in cellular medicine.
Global Stem Cel ls Groups corporate mission is to make the promise of stem cell medicine a reality for patients around the world. With each of GSCGs six operating companies focused on a separate research-based mission, the result is a global network of state-of-the-art stem cell treatments.
ls Groups corporate mission is to make the promise of stem cell medicine a reality for patients around the world. With each of GSCGs six operating companies focused on a separate research-based mission, the result is a global network of state-of-the-art stem cell treatments.
About Adimarket:
Adimarket, Inc., a division of the Global Stem Cells Group, is a cost-competitive online marketplace for quality regenerative medicine equipment and supplies for physicians and health care professionals.
Adimarket was founded to provide physicians and other health care professionals the tools they need to practice regenerative medicine in a medical office setting. Motivated by a firm belief in the impact the practice of stem cell medicine can have when dispensed in a doctor’s office, Adimarket provides physicians with the tools they need to provide patients with cutting edge treatments.
Adimarket’s experienced customer service representatives provide valuable guidance and advice regarding products relevant to individual practices.
About EmCyte:
 Fort Myers, Florida-based EmCyte Corporation is a leader in autologous cellular biologics with the GenesisCS Component Concentrating Systems. These systems provide patients with the best opportunity for rapid recovery and provide practitioners with the most advanced clinical point of care experience. EmCyte systems are developed to meet every clinical requirement, giving the physician better clinical choices. EmCyte devices have been independently reviewed and show to produce buffycoat concentrations of 6x to greater than 10x baseline in 7mLs, with yields ranging from 70 percent to greater than 90 percent.
Fort Myers, Florida-based EmCyte Corporation is a leader in autologous cellular biologics with the GenesisCS Component Concentrating Systems. These systems provide patients with the best opportunity for rapid recovery and provide practitioners with the most advanced clinical point of care experience. EmCyte systems are developed to meet every clinical requirement, giving the physician better clinical choices. EmCyte devices have been independently reviewed and show to produce buffycoat concentrations of 6x to greater than 10x baseline in 7mLs, with yields ranging from 70 percent to greater than 90 percent.
EmCyte technology allows for the safe extraction of concentrated platelets and other regenerative cell types from the patient’s own blood. These cells are then re-suspended in a small volume of the patient’s blood plasma and then applied to the treatment site.
###
- Published in Press Releases
Global Stem Cells Announces Formal Inaugural for Clinica Biomaster in Costa Rica
Global Stem Cells Group has launched Clinica Biomaster Costa Rica with an inauguration and symposium at Clinica Biomaster, the company’s new stem cell center in Escazú, Costa Rica.
Global Stem Cells Group hosted a symposium and formal inauguration of Clinica Biomaster Costa Rica, the company’s new stem cell center in Escazú, Costa Rica. Joseph Purita, M.D., head of the Global Stem Cells Group Scientific Advisory Board, was the keynote speaker at the event, held July 15 and 16, 2016.
Purita also presented lectures to medical staff at Hospital CIMA and Hospital Metropolitano in San Jose during the inaugural weekend.

Joseph Purita, M.D.
The two-day symposium officially launched Global Stem Cells Group’s Costa Rica operations, which includes plans for four stem cell training courses for physicians and a regenerative medicine symposium in early 2017.
Clinica Biomaster is headed by neurologist and anti-aging specialist Dra. Mariella Tanzi, founder of BIOMEN S.A. Tanzi and Biomaster have formed an alliance with GSCG to be the exclusive representative for the Miami-based biomedical company’s products and services in the Costa Rica market.
The symposium included sessions on clinical advances in stem cell research; molecular biology; models of treatment in surgical and cosmetic applications, and in clinical conditions; application of minimally manipulated stem cells in the physician’s office; stem cells, regenerative medicine and its application in anti-aging medicine and medical legal issues. It also included a full day, hands-on training session to provide participating physicians and qualified medical professionals with state-of-the-art techniques for isolating and re-integrating adipose- and bone marrow-derived stem cells for in office patient treatments, along with clinical protocols.
 This popular training course is part of the Global Stem Cells Group’s commitment to the growing network of world-class stem cell researchers, treatment practitioners and investors committed to advancing stem cell medicine, and helping physicians bring treatments into the office for the benefit of patients.
This popular training course is part of the Global Stem Cells Group’s commitment to the growing network of world-class stem cell researchers, treatment practitioners and investors committed to advancing stem cell medicine, and helping physicians bring treatments into the office for the benefit of patients.
To learn more, visit the Global Stem Cells Group website, or the Stem Cell Training website, email bnovas(at)regenestem(dot)com, or call +1 305 560 5337.
About Global Stem Cell Group:
Global Stem Cells Group, Inc. is the parent company of six wholly owned operating companies dedicated entirely to stem cell research, training, products and solutions. Founded in 2012, the company combines dedicated researchers, physician and patient educators and solution providers with the shared goal of meeting the growing worldwide need for leading edge stem cell treatments and solutions. With a singular focus on this exciting new area of medical research, Global Stem Cells Group and its subsidiaries are uniquely positioned to become global leaders in cellular medicine.
To review this press release live online, click here
###
- Published in Press Releases
Global Stem Cells Group Announces Stem Cell Training Course in South Korea
(Pictured: bone marrow stem cells)
Global Stem Cells Group will host a stem cell training course in SVF and bone marrow aspiration techniques July 28-29, 2016.
MIAMI, July 28, 2016–Global Stem Cells Group, in collaboration with South Korean biomedical company N-Biotek will host a course in stromal vascular fraction (SVF) and bone marrow aspiration techniques for physicians at the N-Biotek headquarters in Gyeonggi-do Province of South Korea July 29 and 29, 2016.
The training course is part of a collaborative agreement between GSCG’s Adimarket division and N-Biotek, a worldwide  biomedical and lab equipment manufacturer, to promote and distribute their stem cell technology equipment throughout Latin America.
biomedical and lab equipment manufacturer, to promote and distribute their stem cell technology equipment throughout Latin America.
The two-day, hands-on training covers the latest technology and procedures in SVF and bone marrow stem cell techniques. Practitioners learn skills that can be used to treat patients in their practices, and for career advancement. The SVF and bone marrow aspiration course was developed for physicians and high-level practitioners to learn techniques in harvesting and reintegrating stem cells derived from adipose tissue and bone marrow. The objective of the training teach effective, in-office regenerative medicine techniques.
 N-Biotek develops a range of custom lab products including the Esfomi cosmetic line, and the Stem Cell Total Solution for emerging stem cell businesses.
N-Biotek develops a range of custom lab products including the Esfomi cosmetic line, and the Stem Cell Total Solution for emerging stem cell businesses.
N-Biotek is the only company that builds the whole stem cell processing system for partners ready to begin work in the stem cell industry. N-Biotek meets every need for stem cell clinicians, including biological clean room construction, equipment installation and stem cell processing consulting.
N-Biotek currently distributes medical equipment and services to facilities and professionals in more than 100 countries.
For more information, visit the Global Stem Cells Group website, email bnovas@stemcellsgroup(dot)com, or call +1 305 560 5337.
About Global Stem Cells Group:
Global Stem Cells Group, Inc. is the parent company of six wholly owned operating companies dedicated entirely to stem cell research, training, products and solutions. Founded in 2012, the company combines dedicated researchers, physician and patient educators and solution providers with the shared goal of meeting the growing worldwide need for leading edge stem cell treatments and solutions. With a singular focus on this exciting new area of medical research, Global Stem Cells Group and its subsidiaries are uniquely positioned to become global leaders in cellular medicine.
research, training, products and solutions. Founded in 2012, the company combines dedicated researchers, physician and patient educators and solution providers with the shared goal of meeting the growing worldwide need for leading edge stem cell treatments and solutions. With a singular focus on this exciting new area of medical research, Global Stem Cells Group and its subsidiaries are uniquely positioned to become global leaders in cellular medicine.
About Adimarket:
Adimarket, Inc., a division of the Global Stem Cells Group, is a cost-competitive online marketplace for quality regenerative medicine equipment and supplies for physicians and health care professionals.
Adimarket was founded to provide physicians and other health care professionals the tools they need to practice regenerative medicine in a medical office setting. Motivated by a firm belief in the impact the practice of stem cell medicine can have when dispensed in a doctor’s office, Adimarket provides physicians with the tools they need to provide patients with cutting edge treatments.
Adimarket’s experienced customer service representatives provide valuable guidance and advice regarding products relevant to individual practices.
About N-Biotek:
N-Biotek, Inc., founded in 2000 and located in the Gyeonggi-do Province of South Korea, is a leading manufacturer and supplier of bio-technology-related laboratory equipment. N-Biotek delivers high quality biomedical equipment to more than 100 countries.
To view this press release live online, click here
###
- Published in Press Releases
Regenerating and Restoring Brain Cells in the Aged With Donor Neural Stem Cells
The human brain, as it turns out, is far more malleable than we once thought. Even adult brains. But they are subject to age-related diseases and disorders, such as dementia and diminished cognitive function.
There is hope that medical science may be able to replace brain cells and restore memory in aging patients thanks to new discoveries in neural stem cell techniques. Researchers at the Texas A&M Health Science Center College of Medicine recently published new findings in the journal Stem Cells Translational Medicine that suggests a new technique for preparing donor neural stem cells and grafting them into an aged brain can regenerate tissue that has succumbed to structural, chemical, and functional changes, as well as a host of neurocognitive changes that can be attributed to aging.
The study, titled “Grafted Subventricular Zone Neural Stem Cells Display Robust Engraftment and Similar Differentiation Properties and Form New Neurogenic Niches in the Young and Aged Hippocampus,” was led by Ashok K. Shetty, Ph.D., a professor in the Department of Molecular and Cellular Medicine. associate director of the Institute for Regenerative Medicine, and research career scientist at the Central Texas Veterans Health Care System.
Shetty and his team at Texas A&M focus on the aged hippocampus, which plays an important role in making new memories and connecting them to emotions. They took healthy donor neural stem cells and implanted them into the hippocampus of an animal model, essentially enabling them to regenerate tissue.
The hippocampus in the aging brain
“We chose the hippocampu s because it’s so important in learning, memory and mood function,” Shetty said. “We’re interested in understanding aging in the brain, especially in the hippocampus, which seems particularly vulnerable to age-related changes.”
s because it’s so important in learning, memory and mood function,” Shetty said. “We’re interested in understanding aging in the brain, especially in the hippocampus, which seems particularly vulnerable to age-related changes.”
The volume of this part of the brain seems to decrease during the aging process, and this decrease may be related to age-related decline in neurogenesis (production of new neurons) and the memory deficits some people experience as they grow older.
The aged hippocampus also exhibits signs of age-related degenerative changes in the brain, such chronic low-grade inflammation and increased reactive oxygen species.
Bharathi Hattiangady, assistant professor at the Texas A&M College of Medicine and co-first author of the study said his team was excited to discover that the aged hippocampus can accept grafted neural stem cells as well as the young hippocampus does, a discovery that has significant implications for treating age-related neurodegenerative disorders.
“It’s interesting that even neural stem cell niches can be formed in the aged hippocampus,” Hattiangady says.
 Shetty’s previous research focused on the benefits of resveratrol (an antioxidant that is famously found in red wine and the skin of red grapes, as well as in peanuts and some berries) to the hippocampus. Although the results indicated important benefits for preventing memory loss in aging brains, his newest work demonstrates a way to affect the function of the hippocampus more directly.
Shetty’s previous research focused on the benefits of resveratrol (an antioxidant that is famously found in red wine and the skin of red grapes, as well as in peanuts and some berries) to the hippocampus. Although the results indicated important benefits for preventing memory loss in aging brains, his newest work demonstrates a way to affect the function of the hippocampus more directly.
Neural stem cell grafting
In this new study, the team found that the neural stem cells engrafted well onto the hippocampus in the young animal models (which was expected) as well as the older ones that would be, in human terms, about 70 years old. Not only did these implanted cells survive, they divided several times to make new cells.
“They had at least three divisions after transplantation,” Shetty said. “So the total yield of graft-derived neurons and glia (a type of brain cell that supports neurons) were much higher than the number of implanted cells, and we found that in both the young and aged hippocampus, without much difference between the two.”
 In both old and young brains, a small percentage of the grafted cells retained their stemness feature—an essential characteristic of a stem cell that distinguishes it from ordinary cells—and continuously produced new neurons. This is called creating a new ‘niche’ of neural stem cells, and these niches seemed to be functioning well. They were still producing new neurons at least three months after implantation, and these neurons are capable of migrating to different parts of the brain.
In both old and young brains, a small percentage of the grafted cells retained their stemness feature—an essential characteristic of a stem cell that distinguishes it from ordinary cells—and continuously produced new neurons. This is called creating a new ‘niche’ of neural stem cells, and these niches seemed to be functioning well. They were still producing new neurons at least three months after implantation, and these neurons are capable of migrating to different parts of the brain.
Past efforts to rejuvenate brains using fetal neurons in this way weren’t nearly as successful. Immature cells, such as neural stem cells, seem to do a better job because they can tolerate the hypoxia (lack of oxygen) and trauma of the brain grafting procedure better than post-mitotic or relatively mature neurons. When researchers tried in the past to implant these partially differentiated cells into the aged hippocampus, they didn’t do nearly as well. The research team used a new technique of preparing the donor neural stem cells, which Shetty says is why this result has never been seen before.
Brain marrow
The researchers used donor cells from the sub-ventricular zone of the brain, an area called the “brain marrow,” because it is analogous to bone marrow in that it holds a number of neural stem cells that persist throughout life. These neural stem cells continuously produce new neurons that migrate to the olfactory system. They also respond to injury signals in conditions such as stroke and traumatic brain injury and replace some of the lost cerebral cortical neurons.
Induced pluripotent cells from skin
Even a small stem cell sample is good enough to expand in culture, so the procedure isn’t terribly invasive. However, in the future, a single skin cell might suffice, as similar neural stem cells can be obtained in large numbers from skin. In fact, it is well known in medical science that a number of cells in the body—including skin cells—can be modified in such a way to create induced pluripotent stem cells.
With these cells, scientists can do any number of things, such as making neural stem cells that will make both more of themselves, and make new neurons. It’s not necessary to get the cells from the brain, just take a skin biopsy and push them into neural stem cells, according to Shetty.
Although the way the grafted cells thrived is promising, there is still a good deal of work to be done to determine if the extra grey matter actually improves cognition.
“Next, we want to test what impact, if any, the implanted cells have on behavior and determine if implanting neural stem cells can actually reverse age-related learning and memory deficits,” Shetty said. “That’s an area that we’d like to study in the future.
“I’m always interested in ways to rejuvenate the aged brain to promote successful aging, which we see when elderly persons exhibit normal cognitive function and the ability to make memories.”
###
- Published in Corporate News / Blog
Stem Cell “Tattoo” Technology Allows Researchers to Track Cell Implants Non-invasively
Researchers at the University of Toronto have developed a tracer ink—a “stem cell tattoo”—that provides the ability to monitor stem cells in unprecedented detail after they’re injected.
The research findings, titled “Bifunctional Magnetic Silica Nanoparticles for Highly Efficient Human Stem Cell Labeling,” was published in June in the Journal of Magnetic Resonance Imaging. Already emerging as an ideal probe for noninvasive cell tracking, the technology has the potential to revolutionize stem cell research by arming scientists with the ability to visually follow the pathways and effectiveness of stem cell therapies in the body, in real time.
“Tattoo” tracer can help further development of stem cell therapies
University of Toronto biomedical engineering professor Hai-Ling Margaret Cheng, a biomedical engineer who specializes in medical imaging, says the new technology allows researchers to actually see and track stem cells after they’re injected. Cheng hopes the technique will help expedite the development and use of stem cell therapies.
Working with colleague Xiao-an Zhang, an assistant professor of chemistry at the University of Toronto, Scarborough, Cheng developed a singular chemical compound known as a contrast agent that acts as a tracer. Composed of manganese, an element that naturally occurs in the body, this tracer compound, called MnAMP, bathes stem cells in a green solution, rendering them traceable inside the body under MRI.
Stem cell tracer ink allows long term cell tracking
The contrast agent “ink” first enters a stem cell by penetrating its membrane. Once inside, it stimulates a chemical reaction that prevents it from seeping out of the cell the same way it entered. Previous versions of contrast agents easily escaped cells. By establishing a way to contain the ink within the cell’s walls, the research team achieved the ability to track the cells long term once they are inside the body.

University of Toronto professor Margaret Cheng holds samples of a chemical compound that will create a new way to visualize stem cells inside the body. (Photo: Bernard Weil, Toronto Star)
The thick substance coats the esophagus and other areas of the body with an illuminating compound, making them visible in an x-ray or CT scan. But the barium solution is eliminated from the body within 2 – 3 days or less. Before the stem cell tattoo tracer ink was developed, surgery was the only option for scientists to get a literal glance of a cells’ destiny after it was injected into the body. Now, researchers can track the results in real time, without resorting to any invasive procedures.
“Before, we could not visually track the cells once they were introduced into the body,” Cheng says. “Now we have the ability to view cells in a non-invasive manner using MRI, and monitor them for potentially a very long time.”
Cell tracer technology still in developmental stage
Currently the tracer ink technology is still in the early development phase and requires more animal testing. Cheng is  hopeful it can proceed to human clinical trials in about 10 years. While Cheng has already proven that tattooing an animal’s embryonic stem cell doesn’t affect its ability to transform into a functional heart cell, rat, or even a pig (which better represents a human’s size), larger models are up for evaluation next.
hopeful it can proceed to human clinical trials in about 10 years. While Cheng has already proven that tattooing an animal’s embryonic stem cell doesn’t affect its ability to transform into a functional heart cell, rat, or even a pig (which better represents a human’s size), larger models are up for evaluation next.
In those test cases, researchers will cut off and reduce blood flow in the animals to mimic the effects of damage caused by a human heart attack. Cardiac stem cells pre-tagged with Cheng’s ink tracer technology will then be injected into the damaged tissue. Using MRI to monitor the luminous inked stem cells in action, researchers can non-invasively follow where in the body they’re traveling and more easily determine if the new cells are responsible for restoring normal heart rhythm.
Before it can be tested in humans, the chemical tracer will also have to pass rigorous toxicology tests to ensure its safety.
###
- Published in Corporate News / Blog