Scientists Confirm Reprogrammed Adult Stem Cells Identical to Embryonic Stem Cells
Click on photo (at left) to enlarge
Photo: iPS cells feature – reprogrammed stem cells: Credit: Moscow Institute of Physics and Technology
Russian researchers have concluded that reprogramming does not create differences between reprogrammed and embryonic stem cells.
Stem cells are specialized, undifferentiated cells that can divide and have the remarkable potential to develop into many different cell types in the body during early life and growth. They serve as a sort of internal repair system in many tissues, dividing essentially without limit to replenish other cells. When a stem cell divides, each new cell has the potential either to remain a stem cell or become another more specialized cell type, such as a muscle cell, a red blood cell, or a brain cell. Scientists
distinguish several types of stem cells—pluripotent stem cells can potentially produce any cell in the body. No pluripotent stem cells exist in an adult body, rather they are found naturally in
early embryos.
There are two ways to harvest pluripotent stem cells. The first is to extract them from the excess embryos produced during invitro fertilization procedures, although this practice is still ethically and technically controversial because it does destroy an embryo that could have been implanted. For this reason, researchers came up with the second way to get pluripotent stem cells— reprogramming adult cells.
Reprogramming, the process of “turning on” genes that are active in a stem cell and “turning off” genes that are responsible for cell specialization was pioneered by Shinya Yamanaka, who showed that the introduction of four specific proteins essential during early embryonic development could be used to convert adult cells into pluripotent cells. Yamanaka was awarded the 2012 Nobel Prize along with Sir John Gurdon for the discovery that mature stem cells can be reprogrammed to become pluripotent.
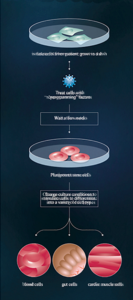
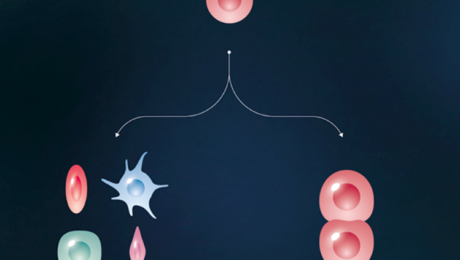
Production of iPS cells:
• Isolate cells from patient; grow in a dish •
Treat cells with “reprogramming”
• Wait a few weeks
• Pluripotent stem cells
• Change culture conditions to stimulate cells to differentiate into a variety of cell types
• blood cells | gut cells | cardio muscle cells
Credit: Moscow Institute of Physics and Technology
However, the extent of the similarity between induced pluripotent stem cells and human embryonic stem cells remains unclear. Recent studies highlighted significant differences between these two types of stem cells, although only a limited number of cell lines of different origins were analyzed.
Researchers compared induced pluripotent stem cell (iPSC) lines reprogrammed from adult cell types that were previously differentiated from embryonic stem cells. All these cells were isogenic, meaning they all had the same gene set.
Scientists analyzed the transcriptome – the set of all products encoded, synthesized and used in a cell. Moreover, they elicited methylated DNA areas, because methylation plays a critical role in cell specialization. Comprehensive studies of changes in the gene activity regulation mechanism showed similarities between reprogrammed and embryonic stem cells. In addition, researchers produced a list of the activity of 275 key genes that can present reprogramming results.
Researchers studied three types of adult cells – fibroblasts, retinal pigment epithelium and neural cells, all of which consist of the same gene set; but a chemical modification (e.g. methylation) combined with other changes determines which part of DNA will be used for product synthesis.
Scientists concluded that the type of adult cells that were reprogrammed and the process of reprogramming did not leave any marks. Differences between cells that did occur were thought to be the result of random factors.
“We defined the best induced pluripotent stem cells line concept,” says Dmitry Ischenko, MIPT Ph.D. and Institute of Physical Chemical Medicine researcher.
The minimum number of iPSC clones that would be enough for at least one to be similar to embryonic pluripotent cells with 95 percent confidence is five.”
 Clearly, no one is going to convert embryonic stem cells into neurons and reprogram them into induced stem cells. Such a process would be too time-consuming and expensive. This experiment simulated the reprogramming of a patient’s adult cells into induced pluripotent stem cells for further medical use, and even though the reprogramming paper, published in the journal Cell Cycle, does not currently propose a method of organ growth in vitro, it is an important step in the right direction. Both induced pluripotent cells and embryonic stem cells can help researchers understand how specialized cells develop from pluripotent cells. In the future, they may also provide an unlimited supply of replacement cells and tissues that can benefit many patients with diseases that are currently untreatable.
Clearly, no one is going to convert embryonic stem cells into neurons and reprogram them into induced stem cells. Such a process would be too time-consuming and expensive. This experiment simulated the reprogramming of a patient’s adult cells into induced pluripotent stem cells for further medical use, and even though the reprogramming paper, published in the journal Cell Cycle, does not currently propose a method of organ growth in vitro, it is an important step in the right direction. Both induced pluripotent cells and embryonic stem cells can help researchers understand how specialized cells develop from pluripotent cells. In the future, they may also provide an unlimited supply of replacement cells and tissues that can benefit many patients with diseases that are currently untreatable.The study, titled, “An integrative analysis of reprogramming in human isogenic system identified a clone selection criterion,” concluded that reprogramming does not create differences between reprogrammed and embryonic stem cells, involved researchers from the Vavilov Institute of General Genetics, Research Institute of Physical Chemical Medicine, and the Moscow Institute of Physics and Technology (MIPT).
###
- Published in Corporate News / Blog
Breakthrough in scaling up life-changing stem cell production
Scientists from the U.K. and Sweden have discovered a new method of creating human stem cells that could solve the problem of meeting large-scale production needs, allowing researchers to fully realize the potential of stem cells for understanding and treating disease.
Human pluripotent stem cells are undifferentiated cells that have the unique potential to develop into all the different types of
cells in the body. With applications in disease modeling, drug screening, regenerative medicine and tissue engineering, there is already an enormous demand for these cells, and that demand will continue grow as their use in clinical settings and the pharmaceutical industry increases.
Human embryonic stem cell line HUES1 grown in the new conditions E8+Inter-alpha-inhibitor and imaged for stem cell marker Oct4 (green) and cell-cell attachment molecule E-cadherin (red) with nuclear counter-staining (blue).
Credit: Dr. Sara Pijuan-Galito and Dr. Cathy Merry, Wolfson Centre for Stem Cells, Tissue Engineering & Modelling and Centre for Biomolecular Sciences, The University of Nottingham
Read more at: http://phys.org/news/2016-07-breakthrough-scaling-life-changing-stem-cell.html#jCp
The research results, published in Nature Communications in July, describe how the scientific team from The University of Nottingham’s Wolfson Centre for Stem Cells, Tissue Engineering and Modelling at Uppsala University in Sweden and GE Healthcare also in Sweden have identified and improved human stem cell culture methods that could lead to quicker and cheaper large scale industrial production of human pluripotent stem cells.
By using a protein derived from human blood called Inter-alpha inhibitor, the team has grown human pluripotent stem cells in a minimal medium without the need for costly and time-consuming biological substrates. Inter-alpha inhibitor is found in human blood at high concentrations, and is currently a by-product of standard drug purification schemes.
The human serum-derived protein can make stem cells attach to unmodified tissue culture plastic, eliminating the need for coating in defined human pluripotent stem cell culture, and improving survival capabilities of the stem cells in harsh conditions.
It is the first stem cell culture method that does not require a pre-treated biological substrate for attachment, and therefore, is more cost and time efficient, paving the way for easier and cheaper large-scale production.
Existing methods are time consuming and make developing human stem cell cultures prohibitively costly. This new method has the potential to save time and money in large-scale and high-throughput cultures, and be highly valuable for both basic research and commercial applications.
The work began at Uppsala University, and the study’s first author, Sara Pijuan-Galitó PhD., is continuing her work as a Swedish Research Council Research Fellow at Nottingham.
Researchers now intend to combine Inter-alpha inhibitor protein with an innovative hydrogel technology to improve on current methods for controlling cell differentiation, and also apply it to disease modelling. The discovery, according to the findings, will help facilitate research into many diseases although their focus is currently on understanding rare conditions like Multiple Osteochondroma) at the cellular level. The aim is to replicate the 3 dimensional environment that cells experience within the body so that lab-bench biology is more accurate in modelling diseases.
 Pijuan-Galitó has been awarded the Sir Henry Wellcome Postdoctoral Fellowship at Nottingham University for her work on the research, which will enable her to combine Inter-alpha inhibitor with improved synthetic polymers in collaboration with fellow regenerative medicine pioneers Professor Morgan Alexander and Professor Chris Denning. The team plans to further improve on current human stem cell culture by designing an economical and safe method that can be easily translated to large-scale production and can deliver billions of stem cells necessary move cellular therapeutics forward in patient settings.
Pijuan-Galitó has been awarded the Sir Henry Wellcome Postdoctoral Fellowship at Nottingham University for her work on the research, which will enable her to combine Inter-alpha inhibitor with improved synthetic polymers in collaboration with fellow regenerative medicine pioneers Professor Morgan Alexander and Professor Chris Denning. The team plans to further improve on current human stem cell culture by designing an economical and safe method that can be easily translated to large-scale production and can deliver billions of stem cells necessary move cellular therapeutics forward in patient settings.The study, titled “Human serum-derived protein removes the need for coating in defined human pluripotent stem cell culture,” was published in Nature Communications in July, 2016.
###
- Published in Corporate News / Blog
Purest Liver-like Cells to Date Generated from Induced Pluripotent Stem Cells (iPSCs)
Researchers from the Medical University of South Carolina (MUSC) and the University of Pennsylvania have discovered a new methodology for purifying liver cells generated from induced pluripotent stem cells (iPSCs) that could facilitate progress toward an important clinical goal: treating patients with disease-causing liver mutations by transplanting unmutated liver cells derived from their own stem cells.
This new technique follows previous attempts to generate liver-like cells from stem cells, which have yielded heterogeneous cell populations with little similarity to diseased livers in patients.
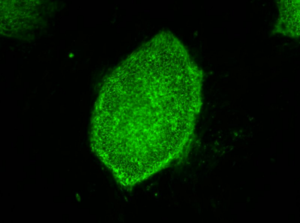
Image: induced pluripotent stem cells expressing a characteristic cell surface protein called SSEA4 (green).
Credit: Image courtesy of Stephen A. Duncan, Ph.D., at the Medical University of South Carolina
The GWAS studies map the genomes in hundreds of people as a way to look for genetic mutation patterns that differ from the genomes of healthy individuals. As GWAS study map more genomes, they become more likely to find the correct genetic mutations that cause a disease. Once a panel of suspected mutations is built, stem cells from these individuals can be manipulated in culture dishes to differentiate into any of the body’s cells. The cells can be screened to learn more about the mutations and to test panels of drugs that might ultimately help treat patients harboring a disease.
Problems arise during the cell manipulation process. For example, iPSCs persistently refuse to mature uniformly into liver-like cells when fed growth factors. Traditionally, antibodies have been used to recognize features of maturity on the surfaces of cells and purify cells that are similar, an approach that has been crucial to stem cell research. But available antibodies that recognize mature liver cells are scanty and tend to recognize many different kinds of cells. The many types of cells in mixed populations have diverse characteristics that can obscure underlying disease-causing genetic variations, which tend to be subtle.
“Without having a pure population of liver cells, it was incredibly difficult to pick up these relatively subtle differences caused by the mutations, but these differences are important in the life of an individual,” Duncan says.
Instead of relying on antibodies, Duncan and his team embraced a new technology called chemo proteomic cell surface capture (CSC) technology. CSC technology allowed the researchers to map the most highly produced proteins on the surface of liver cells during the final stages of differentiation of stem cells into liver cells. The most abundant protein was targeted with an antibody labeled with a fluorescent marker and used to sort the mature liver cells from the rest.
The procedure was highly successful: The team had a population of highly pure, homogeneous and mature liver-like cells. Labeled cells had far more similar traits of mature hepatocytes than unlabeled cells. Pluripotent stem cells that had not differentiated were excluded from the group of labeled cells.
“That’s important,” says Duncan. “If you’re wanting to transplant cells into somebody that has liver disease, you really don’t want to be transplanting pluripotent cells because pluripotent cells form tumors called teratocarcinomas.”
 Duncan cautioned that transplantation of iPSC-derived liver cells is not yet ready for translation to the clinic, but the technology for sorting homogeneous liver cells can be used now to successfully and accurately model and study disease in the cell culture dish.
Duncan cautioned that transplantation of iPSC-derived liver cells is not yet ready for translation to the clinic, but the technology for sorting homogeneous liver cells can be used now to successfully and accurately model and study disease in the cell culture dish.“We think that the ability to generate pure populations will get rid of the variability, and therefore really help us combine with GWAS studies to identify allelic variations that are causative of a disease, at least in the liver,” he says.
Researchers at the University of Minnesota (Minneapolis) and the Medical College of Wisconsin (Milwaukee) contributed to the study, published August 25, in Stem Cell Reports.
###
- Published in Corporate News / Blog
Global Stem Cells Group to offer Stem Cell Training Certification course Oct. 1 and 2, Following International Symposium in Buenos Aires
MIAMI, Sept. 13, 2016-Global Stem Cells Group announced plans to hold a stem cell training certification course Oct. 1 -2, 2016 following the 3rd annual Global Stem Cells Group International Symposium on Stem Cells and Regenerative Medicine in Buenos Aires. The symposium will take place Sept. 28, 2016.
The two-day, hands-on training course covers the latest technology and procedures in SVF and bone marrow stem cell techniques. Practitioners learn skills that can be used to treat patients in their practices, and for career advancement. The SVF and bone marrow aspiration course was developed for physicians and high-level practitioners to learn techniques in harvesting and rein

Joseph Purita, MD

Silvina Pastrana, M.D.
The stem cell training certification course will be held at the Global Subsidiary Stem Cell Center Network in Puerto Madero Buenos Aires immediately following the 3rd annual International Symposium on Stem Cells and Regenerative Medicine.
Since 2014, Global Stem Cells Group has joined forces with some of the most prestigious regenerative medicine practitioners in South America as it focuses on growing its services throughout the global community. Stem cell therapies continue to revolutionize the anti-aging aesthetics industry and help improve the quality of life for patients suffering from some chronic conditions.
To learn more about the 3rd Annual International Symposium on Stem Cells and Regenerative Medicine, visit the Global Stem Cells Group website, email bnovas(at)stemcellsgroup(dot)com, or call 305-560-5337.

About Global Stem Cells Group:
Global Stem Cells Group, Inc. is the parent company of six wholly owned operating companies dedicated entirely to stem cell research, training, products and solutions. Founded in 2012, the company combines dedicated researchers, physician and patient educators and solution providers with the shared goal of meeting the growing worldwide need for leading edge stem cell treatments and solutions. With a singular focus on this exciting new area of medical research, Global Stem Cells Group and its subsidiaries are uniquely positioned to become global leaders in cellular medicine.
To view this press release live online, click here
###
- Published in Press Releases
Global Stem Cells Group to Attend the First Latin American Congress on Sports Medicine in Panama
MIAMI, Sept. 9, 2016—Global Stem Cells Group will attend the Colombian Association of Sports Medicine’s First Latin American Congress on Sports Medicine, Injuries, Treatment and Rehabilitation in Panama City, Panama Sept. 22 – 24, 2016. The Panama Congress will focus on a variety of Sports Medicine specialties presented in an academic activity framework.
The congress will host a diverse range of esteemed guests, including physicians, athletes, athletic coaches, physical therapists, medical exercise physiologists, orthopedists, physical medicine and rehabilitation (PM&R) physicians (physiatrists), Paralympics athletes, endocrinologists, diabetologists, orthopedic surgeons, internists, physical educators, pain specialists, nutrition and dietetic specialists, psychologists, neuro surgeons and neuro rehabilitation specialists.
The event, to be hosted at the Hotel Riu Plaza in the heart of Panama City’s financial district, will provide a dynamic atmosphere showcasing local, national, international and Latin American ambience.
To learn more about the First Latin American Congress on Sports Medicine, visit the event website.To learn more about Global Stem Cells Group, visit the GSCG website, email bnovas(at)stemellsgroup.com, or call 305-560-5337.

About Global Stem Cells Group:
Global Stem Cells Group, Inc. is the parent company of six wholly owned operating companies dedicated entirely to stem cell research, training, products and solutions. Founded in 2012, the company combines dedicated researchers, physician and patient educators and solution providers with the shared goal of meeting the growing worldwide need for leading edge stem cell treatments and solutions. With a singular focus on this exciting new area of medical research, Global Stem Cells Group and its subsidiaries are uniquely positioned to become global leaders in cellular medicine.
To review this press release live online, click here
###
- Published in Press Releases
Global Stem Cells Group Announces Speaker Lineup for the 3rd Annual International Symposium on Stem Cells and Regenerative Medicine
MIAMI, Sept. 9, 2016—Global Stem Cells Group has announced its lineup of speakers for the 3rd Annual International Symposium on Stem Cells and Regenerative Medicine, to be held Sept. 28, 2016 in Buenos Aires, Argentina. In keeping with tradition, the symposium will be hosted by GSCG in collaboration with Julio Ferreira, M.D., President of the South American Academy Cosmetic Surgery.
The symposium will be a gathering of many of the world’s most respected authorities on stem cell and regenerative medicine, who will share the latest findings and showcase advancements in research and therapies on a global level. The interdisciplinary team of leading international stem cell experts will provide a full day of high-level scientific lectures geared to medical professionals. Pioneers and luminaries in stem cell medicine who will serve as featured speakers at the event include:

Joseph Purita, M.D., USA
Musculoskeletal and antiaging applications of stem cells.
Joseph Purita, M.D., is an internationally recognized orthopedic surgeon and pioneer in the application of stem cells and PRP therapies for orthopedic injuries and diseases. Dr. Purita pioneered laser techniques in orthopedic surgery, and specializes in the treatment of high-performance athletes. Dr. Purita is a member of the Global Stem Cells Group Advisory Board.
[su_spacer]
 Enrique Testart,, M.D., Chile
Enrique Testart,, M.D., Chile
Osteogenesis and the future of heterologous implants.
Enrique Testart, M.D., is a pediatric surgeon specializing in child trauma microsurgery. Dr. Testart has developed distinctive techniques and protocols in cellular therapies and management protocols for musculoskeletal disorders. He has dedicated years to researching and practicing techniques that employ stem cell therapies to enhance conventional surgical techniques to ease patient suffering. Dr. Testart is Global Stem Cells Group’s Chile Representative
 Rafael Pérez Franco, M.D., Colombia
Rafael Pérez Franco, M.D., Colombia
Use of cell therapy in the treatment of alopecia.
Rafael Perez Franco, M.D. is director of the Rafael Perez Center for Reconstructive and Aesthetic Surgery in Bogota, Colombia. An expert in his field for more than 10 years, Dr. Perez Franco is a surgeon at the Universidad del Bosque and a plastic surgeon at the Clínica San Rafael in Colombia. Dr. Perez Franco also holds special rotations at Hospital Saint Paul in San Pablo, Brazil and the Clínica Fluminense de Cirugía Plástica and Clínica del Profesor Ivo Pitanguy in Rio de Janeiro. He is a member of the Global Stem Cells Group Advisory Board.
 Carlos Chiriboga Assini, M.D., Ecuador
Carlos Chiriboga Assini, M.D., Ecuador
Application of Neural therapy and stem cell therapy in the treatment of injuries for high performance athletes.
Carlos Chirigoba Assini, M.D. serves as Chairman of the Board ar Omnihospital Medical Association in Guayaquil, Ecuador. Dt. Chirigoba Assini specializes in traumatology, orthopedic surgery, and platelet rich plasma (PRP) therapies. He is a member of Omnihospital Medical Association and the American Academy of Antiaging.
 Aldo Parodi, M.D. Perú
Aldo Parodi, M.D. Perú
Aesthetic applications of cellular therapies.
Aldo Parodi, M.D. is a plastic surgeon and laser liposuction specialist with clinical experience in assisted lipotransference. Dr. Parodi offers aesthetic treatments using adipose-derived stem cells at his aesthetic medicine clinic Clinic La Femme in Tacna, Peru. He is a member of the Global Stem Cells Group Advisory Board.
[su_spacer]
 Silvina Pastrana, M.D. Argentina.
Silvina Pastrana, M.D. Argentina.
Implementation of cellular therapies. Presentation of clinical cases.
Silvina Pastrana, M.D. is a surgeon and medical director of the Stem Cell Center Buenos Aires and Regentherapy Puerto Madero. Dr. Pastrana heads a staff of medical specialists in orthopedics, rheumatology, medical clinic and cosmetic surgery, performing procedures incorporating stem cell therapies. She also serves as a staff surgeon for the Hospital Dr. Prof. Luis Güemes, and is a member of the Global Stem Cells Group Advisory Board.
 Benito Novas, CEO, Global Stem Cell Group, United States
Benito Novas, CEO, Global Stem Cell Group, United States
Developments and Challenges of Regenerative medicine globally.
Benito Novas, founder, CEO and creative director of Global Stem Cells Group, Mr. Novas also spearheaded and launched Stem Cell Training Inc. to provide professional training courses in stem cell therapies for physicians. Mr. Novas oversees Stem Cell Training marketing programs, finance strategies, field operations administration, policies, procedures and accreditation. Prior to founding The Global Stem Cells Group, Mr. Novas was President and CEO of Adimarket, Inc.
 Leonardo Tacus, M.D. Argentina
Leonardo Tacus, M.D. Argentina
Muscle and tendon injuries in regenerative medicine.
Leonardo Tacus, M.D. is an arthroscopic surgeon specializing in orthopedics, traumatology, and regenerative medicine. Dr. Tacus is the medical director at iar sa Argentina Hospital and Health Care, and co-director of Education Center Graduate Medical Surgery, orthopedics and Trauma (CECOA) in Buenos Aires. He is a founding member of the Argentina Association of Arthroscopy and past secretary of the Argentina Association of Arthroscopy
 Daniel Domínguez, Aregntina
Daniel Domínguez, Aregntina
Systematics in the preparation of blood components for Regenerative Medicine.
Daniel Domínguez is the operations manager of High Technology Systems S.A. in Buenos Aires. Mr. Dominguez’s background includes hemotherapy and laboratory tech.
 Susana Miriam Gjurkan, D.M.D. Argentina
Susana Miriam Gjurkan, D.M.D. Argentina
Application of stem cells and PRP in temporomandibular joint ATM (modified technique)
Susana Miriam Gjurkan, D.M.D., is a surgical specialist in orthodontics, cosmetic dentistry, dental implants, preventive dentistry, pediatric dentistry, periodontics, prosthetic dentistry, neurofacial and aesthetic dentistry. She uses stem cells and PRP to treat temporomandibular joint (TMJ) disorders in patients (modified technique). Dr. Gjurkan operates from her clinic, ONE Surgery, in San Miguel de Tucuman, Argentina. She has attended and taught courses in neural therapy, and earned her stem cell certification through Stem Cell Training.
Since 2014, Global Stem Cells Group has joined forces with some of the most prestigious regenerative medicine practitioners in South America as it focuses on growing its services throughout the global community. Stem cell therapies continue to revolutionize the anti-aging aesthetics industry while offering new hope for sufferers of serious chronic debilitating diseases.
To learn more about the 3rd Annual International Symposium on Stem Cells and Regenerative Medicine, visit the Global Stem Cells Group website,email bnovas(at)stemcellsgroup(dot)com, or call 305-560-5337.
About Global Stem Cells Group:
Global Stem Cells Group, Inc. is the parent company of six wholly owned operating companies dedicated entirely to stem cell research, training, products and solutions. Founded in 2012, the company combines dedicated researchers, physician and patient educators and solution providers with the shared goal of meeting the growing worldwide need for leading edge stem cell treatments and solutions. With a singular focus on this exciting new area of medical research, Global Stem Cells Group and its subsidiaries are uniquely positioned to become global leaders in cellular medicine.
About Julio Ferreira, M.D.

Julio Ferreira, M.D., President of the South American Academy of Cosmetic Surgery, is a professor at the Institute of Biomedical Sciences, University of Sao Paulo, Brazil. In addition, he is the former President of the International Academy of Cosmetic Surgery 2005/2007; a member and examiner, International Board of Cosmetic Surgery; Corresponding Fellow of the American Academy of Cosmetic Surgery; Honorary Member of the Spanish Society of Cosmetic Medicine and Surgery; Honorary Member of the Eurorusa Confederation of Societies of Aesthetic Plastic Surgery; Honorary Member of the Bulgarian Society of Cosmetic Surgery; Honorary Member of the Chilean Society of Cosmetic Surgery and Lipoplasty; Honorary Member of the Italian Society of Aesthetic Surgery; Honorary Member of the French Society of Aesthetic Surgery; Honorary Member of the Japan Society of Aesthetic Surgery; Honorary Member of the Ecuadorian Society of Medical Aesthetics; Honorary Member of the Peruvian Society of Cosmetic Surgery, and a member of the International Editorial Advisory Board of the American Journal of Cosmetic Surgery.
To view this press release live online, click here
###
- Published in Press Releases
Global Stem Cells Group Subsidiary Adimarket Announces New Closed System Kit for Isolation of Adipose Derived Stem Cells, Development by N-Biotek
MIAMI, Sept.8, 2016—Adimarket, a subsidiary of Global Stem Cells Group, Inc., has announced that Korean biotech leader N-Biotek is developing a new closed system kit containing all of the elements necessary to process adipose tissue and obtain stromal vascular fraction (SVF) in a closed environment.
Created with N-Biotek technology, the system will allow entire procedures to be performed in a sterile closed system.
Adipose derived stem cells (ASCs) are used by physicians for a variety of indications.
Most commonly, ASCs are isolated at the point of care from lipoaspirate (derived from liposuction) tissue as the stromal vascular fraction (SVF), harvested from the patient and immediately administered to the patient as an injection, or used to enrich fat grafts. Isolation of ASCs from adipose tissue is a relatively simple process performed routinely in cell biology laboratories, but isolation at the point of care for immediate clinical administration requires special methodology to prevent contamination, ensure integrity of the clinical procedure, and comply with regulatory requirements.
Once available on the Adimarket product website, the N-Biotek kit can provide a low cost, rapid and simple alternative to traditional methods of isolating ASCs, particularly when smaller quantities are needed.
To learn more about the N-Biotek closed system kit, visit the Adimarket website or the Global Stem Cells website, email bnovas@stemcellsgroup.com, or call 305-560-5337.
About Global Stem Cells Group:
Global Stem Cells Group, Inc. is the parent company of six wholly owned operating companies dedicated entirely to stem cell  research, training, products and solutions. Founded in 2012, the company combines dedicated researchers, physician and patient educators and solution providers with the shared goal of meeting the growing worldwide need for leading edge stem cell treatments and solutions.
research, training, products and solutions. Founded in 2012, the company combines dedicated researchers, physician and patient educators and solution providers with the shared goal of meeting the growing worldwide need for leading edge stem cell treatments and solutions.
With a singular focus on this exciting new area of medical research, Global Stem Cells Group and its subsidiaries are uniquely positioned to become global leaders in cellular medicine.
Global Stem Cells Groups corporate mission is to make the promise of stem cell medicine a reality for patients around the world. With each of GSCGs six operating companies focused on a separate research-based mission, the result is a global network of state-of-the-art stem cell treatments.
About Adimarket:
Adimarket, Inc., a division of the Global Stem Cells Group, is a cost-competitive online marketplace for quality regenerative medicine equipm ent and supplies for physicians and health care professionals. Adimarket
ent and supplies for physicians and health care professionals. Adimarket
was founded to provide physicians and other health care professionals the tools they need to practice regenerative medicine in a medical office setting. Motivated by a firm belief in the impact the practice of stem cell medicine can have when dispensed in a doctor’s office, Adimarket provides physicians with the tools they need to provide patients with cutting edge treatments.
Adimarket’s experienced customer service representatives provide valuable guidance and advice regarding products relevant to individual practices.
About N-Biotek:
 Since 1982, N-Biotek has been the leading manufacturer of biomedical and lab equipment worldwide. The Stem Cell Total Solution lab is a culmination of years of dedication to establish compact and customized products in developing the Handy Lab, a personal lab for medical, scientific and university professionals. N-Biotek is known for its high quality, uniquely designed biomedical products and equipment and competitive pricing. Over the years, the company’s products have earned an array of patents, and all meet international standards including CE, ETL, ISO and GMP. N-Biotek also offers real-time monitoring through IT technology.
Since 1982, N-Biotek has been the leading manufacturer of biomedical and lab equipment worldwide. The Stem Cell Total Solution lab is a culmination of years of dedication to establish compact and customized products in developing the Handy Lab, a personal lab for medical, scientific and university professionals. N-Biotek is known for its high quality, uniquely designed biomedical products and equipment and competitive pricing. Over the years, the company’s products have earned an array of patents, and all meet international standards including CE, ETL, ISO and GMP. N-Biotek also offers real-time monitoring through IT technology.
Since 2010, N-Biotek has expanded significantly, starting new businesses including a stem cell processing system and biological clean room, GMP consulting, validation services and health care services for foreign consumers in order to maintain its lead in the life science field.
To view this press release live online, click here
###
- Published in Press Releases
Benito Novas, CEO, Global Stem Cells Group
Benito Novas, founder, CEO and creative director of Global Stem Cells Group. Mr.Novas spearheaded and launched Stem Cell Training Inc. to provide professional training courses in stem cell therapies for physicians.
Mr. Novas oversees Stem Cell Training’s marketing programs, finance strategies, field operations administration, policies, procedures and accreditation. Prior to founding The Global Stem Cells Group, Mr. Novas was President and CEO of Adimarket, Inc.
A critical thinker and analytical problem solver with solid relationship building skills, Mr. Novas is a proven leader and team builder who is able to manage, motivate, and train staff.
Most recently, Mr. Novas co-authored a book with Catherine Maley from Cosmetic Image Marketing titled, “Your Aesthetic Practice: A Complete Guide: What Your Patients Are Saying.” Catherine Maley and Benito Novas work closely together to help aesthetic and cosmetic practitioners worldwide.
- Published in Advisory Board Members
Global Stem Cells Group Announces Permanent Stem Cell Training Center in South Korea
MIAMI, Aug. 30, 2016 – Global Stem Cells Group and its subsidiary Stem Cell Training, Inc., in a collaborative agreement with South Korean biomedical company N-Biotek, announce the establishment of a permanent stem cell training center at the N-Biotek headquarters in Seoul, South Korea. The announcement comes as part of an ambitious expansion effort between GSCG and N-Biotek to provide stem cell training to qualified physicians worldwide.
Stem Cell Training’s two-day intensive “Adipose-derived Stem Cell and Bone Marrow and Platelet Rich Plasma (PRP)” training course covers concepts in cellular medicine, cell viability basics, basic fat and bone marrow stem cell harvesting, isolation and processing procedures, and principles of platelet rich plasma (PRP) processing.
The Korean training center will offer GSCG’s full array of Stem Cell Training courses including:
The “Cell Assisted Fat Transfer” training course is designed for physicians in the aesthetic and cosmetic fields of medicine. The course specifically focuses on procedures to address facial aging caused by volume loss. Course training covers the basics of facial anatomy, facial aging, and the use of fat harvested from the patient’s own body as a filler.
The “Cell Assisted Fat Transfer” training prepares the physician to perform a mini liposuction procedure in order to retrieve fat for grafting in the patient’s anti-aging treatment. It also provides instruction on placement techniques for female and male patients according to age and ethnicity. Fat is a popular filler in the aesthetic medicine field, as the industry experiences an increase in liposuction procedures, an increased interest in facial re-volumization and body, and a growing appreciation for the regenerative potential of adipose-derived mesenchymal stem cells.
The “Diplomat Cell Therapy and Tissue Engineering” course is offered to physicians and qualified practitioners interested in implementing regenerative medicine therapies. This course offers a detailed, hands-on experience in stem cell characterization and laboratory applications; cell protocols including culture, plating, trypsinization, harvesting and cryopreservation; instruction in applying quality control tests including cell count, viability, flow cytometry, endotoxin, mycoplasma and sterility; the ability to perform cGMP functions including clean room maintenance, gowning and environmental monitoring, and new insight and relevant applications of stem cell processing and regulations in a certified facility.
The “Online SVF and Bone Marrow” training course is an online stem cell training course that provides physicians an opportunity to take the clinical training course in a web based format presented as video and voiced over slides. The online course content is consistent with Stem Cell Training’s clinical training course, as it focuses on autologous cellular treatments such as adult stem cells and platelet rich plasma (PRP). Attendees learn how to extract lipoaspirate and bone marrow aspirate, and how to isolate adipose and bone marrow derived stem cells.
In addition, all techniques and materials presented in the training videos are accompanied with detailed protocols.
This course also provides attendees with the tools necessary to implement regulatory and clinical guidelines when setting up a GMP facility; copies of presentations, procedural protocols and all forms associated with a GMP facility; the ability to perform clinical procedures including lipoaspirate and bone marrow isolation; the ability to reintroduce stem cells for various indications, and case books and full protocols for approximately 30 indications.
The two-day online clinical course includes in-clinic procedural videos, recorded didactic lectures, additional information and videos on regenerative medicine, and digital versions of course procedures, protocols and videos.
Stem Cell Training’s experienced and knowledgeable trainers will cover indications, contraindications, patient selection, pre- and post-treatment instructions, and treatment alternatives for autologous facial fat transfer. In addition, participants will learn to analyze anatomy for appropriate areas of facial enhancement; learn various injection techniques to correct temporal hollows, volume loss, tear trough deformity, pre jowl sulcus, malar fat pad, submalar areas, and melomental and nasolabial folds; learn to revent and manage cell-assisted autologous facial fat transfer complications, and understand anesthetic techniques.
To learn more about the stem cells training course center in South Korea, visit the Global Stem Cells Group website, or the Stem Cell Training website, email bnovas@stemcellsgroup.com, or call +1 305 560 5337
About Global Stem Cell Group:
Global Stem Cells Group is the parent company of six wholly owned operating companies dedicated entirely to stem cell research, training, products and solutions. Founded in 2012, the company combines dedicated researchers, physician and patient educators and solution providers with the shared goal of meeting the growing worldwide need for leading edge stem cell treatments and solutions. With a singular focus on this exciting new area of medical research, Global Stem Cells Group and its subsidiaries are uniquely positioned to become global leaders in cellular medicine.
 About Stem Cell Training, Inc.:
About Stem Cell Training, Inc.:
Stem Cell Training, Inc. is a multi-disciplinary company offering coursework and training in 35 cities worldwide. The coursework offered focuses on minimally invasive techniques for harvesting stem cells from adipose tissue, bone marrow and platelet-rich plasma. By equipping physicians with these techniques, the goal is to enable them to return to their practices, better able to apply these techniques in patient treatments.
About N-Biotek:
Since 1982, N-Biotek has been the leading manufacturer of biomedical and lab equipment worldwide. The Stem Cell Total Solution lab is a culmination of years  of dedication to establish compact and customized products in developing the Handy Lab-a personal lab for medical, scientific and university professionals. N-Biotek is known for its high quality, uniquely designed biomedical products and equipment and competitive pricing. Over the years, the company’s products have earned an array of patents, and all meet international standards including CE, ETL, ISO and GMP. N-Biotek also offers real-time monitoring through IT technology.
of dedication to establish compact and customized products in developing the Handy Lab-a personal lab for medical, scientific and university professionals. N-Biotek is known for its high quality, uniquely designed biomedical products and equipment and competitive pricing. Over the years, the company’s products have earned an array of patents, and all meet international standards including CE, ETL, ISO and GMP. N-Biotek also offers real-time monitoring through IT technology.
Since 2010, N-Biotek has expanded significantly, starting new businesses including a stem cell processing system and biological clean room, GMP consulting, validation services and health care services for foreign consumers in order to maintain its lead in the life science field.
To view this press release live online, click here
###
- Published in Press Releases
Global Stem Cells Group Expects Record Attendance at 3rd Annual International Symposium on Stem Cells and Regenerative Medicine in Buenos Aires
Buenos Aires stem cell symposium scheduled for Sept. 28, 2016.
MIAMI, Aug. 30, 2016 – Global Stem Cells Group, in conjunction with Julio Ferreira, M.D., President of the South American Academy Cosmetic Surgery, will host the 3rd annual Global Stem Cells Group International Symposium on Stem Cells and Regenerative Medicine Sept. 28, 2016. The event, expected to attract a record number of physicians, researchers and regenerative medicine experts from around the world, offers an opportunity for many of the world’s most respected authorities on stem cell and regenerative medicine to showcase advancements in research and therapies on a global level.

Enrique Testart, M.D.
According to Benito Novas, Global Stem Cells Group CEO, the world-class event will showcase the historic advances stem cell medicine has achieved since the first symposium was held just two years ago in 2014.

Joseph Purita, MD
“This year, we will be able to showcase how far stem cell therapies have come since then, and provide some of the most influential leaders that understand the potential of these therapies and have dedicated their careers to making them a reality.”

Sylvina Pastrana, M.D.
To learn more about the 3rd Annual International Symposium on Stem Cells and Regenerative Medicine, visit the Global Stem Cells Group website, email bnovas@stemcellsgroup.com, or call 305-560-5337.
About Global Stem Cells Group:
Global Stem Cells Group, Inc. is the parent company of six wholly owned operating companies dedicated entirely to stem cell research, training, products and solutions. Founded in 2012, the company combines dedicated researchers, physician and patient educators and solution providers with the shared goal of meeting the growing worldwide need for leading edge stem cell treatments and solutions. With a singular focus on this exciting new area of medical research, Global Stem Cells Group and its subsidiaries are uniquely positioned to become global leaders in cellular medicine.
About Julio Ferreira, M.D.
Julio Ferreira, M.D., President of the South American Academy of Cosmetic Surgery, is director of Clinica Ferreira in Argentina and a professor at the Institute of Biomedical Sciences, University of Sao Paulo, Brazil.
Julio Ferreira, M.D., is an internationally recognized and respected cosmetic surgeon and professor of medicine and aesthetic surgery at the Institute of Biomedical Sciences, University of Sao Paulo, Brazil;
As director of Clinica Ferreira in Argentina, Dr. Ferreira is dedicated to the combination of art and science in aesthetic medicine.
To view this press release live online, click here
###
- Published in Press Releases